Blood Products and Transfusion

Take home messages
- Anaemia and blood transfusion are independent risk factors for postoperative morbidity and mortality
- Don't forget simple but effective things like early administration of tranexamic acid
- Any transfusion reactions need to be reported
Podcast episode
This will blow your mind
This might surprise you, dear reader, but the vast majority of the research seems to suggest that patients do substantially better when:
- They have enough blood in their blood vessels
- They don't lose too much blood
- They receive blood when they need it, and not when they don't
There you go. All of blood product management done.
You can go home now.
What to do with an acutely haemorrhaging patient
- ABCDE and get some big bilateral IV or IO access
- Call for help - you're going to need more hands
- 100% oxygen - to fill up the remaining haemoglobin while you fix things
- Call 2222 and put out Major Haemorrhage call
- Fluid bolus of 250-500ml warm crystalloid while waiting for blood, to maintain a perfusing blood pressure (but don't push the BP above 100-120 systolic)
- Tranexamic acid 1g IV
- Keep the patient warm
- Take an arterial blood gas for a baseline Hb, calcium and lactate
- Get calcium ready (gluconate or chloride) to give once you're infusing red cells - aim to give around 1g per 6 units of PRC targetting ionised Ca of >1 mmol/litre
- Give RBC, FFP and Platelets according to your Trust's protocol
- If you don't know what this is then give 1:1:1:1 PRC:FFP:Platelets:Cryo if you can
- When you get a minute, send of FBC, INR, Clotting, Fibrinogen, U+E
- Use TEG/ROTEM if you have it
A Spot of History
Meet William Harvey.

In 1628 this chap figured out that the blood goes round and round unless the pump stops working or there's a hole somewhere, and shortly afterwards the first blood transfusion was attempted, albeit not particularly successfully.
The first successful transfusion wasn't until 1665 by Richard Lower, who managed to keep a dog alive by transfusing blood into it from several other dogs.
Presumably the 'success' refers to the first dog.
Two years later, Lower cracks on and starts piling lambs' blood into human patients, as does Jean-Baptiste Denis in France - with a predictable mortality - and a sensible subsequent ban on transfusing animal blood into humans is instituted.
It's not until 1795 before American physician with the fabulous surname of Physick manages to perform the first human-to-human transfusion.
Then in 1818 British obstetrician James Blundell managed a woman with post partum haemorrhage by dragging 'four ounces' of blood from her - presumably willing - husband and managed to safe her life.
Clearly boldened by the steadily increasing success rates, physicians in the USA began transfusing milk into humans, however this unsurprisingly caused a fair few reactions, so it was replaced by saline in 1884.
In 1900, Austrian Karl Landsteiner discovers the three main blood groups and his colleagues add AB two years later.
In 1908, inventive Frenchman Alexis Carrel decides to shortcut the whole process and stitch the donor's vein directly to the patient's artery. It didn't work. But it did generate interest in anastomosis and eventually made organ transplant possible.
Here's our post on other things that kill people, if you're interested, and here's some more about the history of blood transfusion.
Prevention is better than cure
When blood falls out - be that as a result of trauma, a surgeon or a medical problem such as peptic ulceration - the patient rather promptly starts losing the ability to send enough oxygen and fuel to the brain and other vital organs.
Three things need to happen, and ideally in the following order:
- Turn off the tap
- Fill them back up
- Deal with any complications of the bleeding, the tap-turning or the filling
The aim of the game, clearly, is to try and avoid transfusion wherever possible, and the unimaginative title given to this practice is 'Patient Blood Management'.
What are the aims of patient blood management?
- Detect and treat preoperative anaemia well before surgery
- Minimise blood loss before, during and after surgery
- Physiological optimisation to enable the patient to cope with blood loss
- Coordinate medical, surgical and anaesthetics teams so everyone is on the same page
Preoperative Anaemia
If your patient is having an operation and is at risk of bleeding, then being anaemic to begin with is a bad start.
What is anaemia?
- A condition wherein the quantity of erythrocytes and their resulting oxygen carrying capacity is insufficient to meed the body's physiological requirements
- Usually defined as Hb <130 g/L in men and <120 g/L in women
- It can be microcytic, normocytic or macrocytic, or a combination of these depending on the cause(s) of the problem
The ESA recommend that Hb be measured between 1-2 months before elective procedures that are likely to involve substantial bleeding, to allow preoperative anaemia to be corrected in good time.
Iron Deficiency
One of the more common reasons for a patient to have a microcytic anaemia is iron deficiency.
What are the causes of iron deficiency anaemia?
- Depletion of total iron stores in the body
- Chronic blood loss
- Malmetabolism of iron in specific disease states such as inflammation and malignancy
Enter Hepcidin
If you've got a lot of iron hanging around and you throw some inflammation into the mix, you start churning out a load more hepcidin.
- This is a hormone that inhibits absorption of iron in the gut by breaking down ferroportin (a transmembrane transporter) in the duodenum.
- It also stops the transport of stored iron from the liver and white cells into the plasma.
So, even if there's physically enough iron hanging around, you can be functionally iron deficient as it can't be used effectively.
This is what is usually meant by 'anaemia of chronic disease'.
Managing preoperative iron deficiency anaemia
- FBC 4-6 weeks before elective surgery
- If anaemic then they need haematinics
- Oral iron is cheap but patients hate it, so compliance is often low
- Its bioavailability is also only 10-15% and it causes hepcidin upregulation that reduces absorption even more
- If you then add in malignancy or gut inflammation, they often don't absorb anywhere near enough
- IV iron is also effective, with fewer GI side effects than oral
- In specific circumstances, erythropoietin can also be used to stimulate RBC production preoperatively
Minimising Blood loss
This is the crucial bit of the equation - minimising how much blood actually leaves the patient's vasculature - and you need to do two things as a team:
- Identify who is at risk of bleeding significantly
- Employ as many anaesthetic and surgical techniques as possible to reduce bleeding
Identify those at risk of bleeding
- Trauma
- Haemophilia
- Menorrhagia
- Family history of bleeding problems
- Anticoagulants
- Liver disease
How to manage anticoagulants in the perioperative period
Google it.
Seriously - patients are on all sorts of new anticoagulants and antiplatelets and the guidance changes - so having a quick look at the latest guidelines is never a bad idea, just make sure you're using a reputable source.
- There are increasing numbers of patients that are taking anticoagulants or antiplatelets (or both) throughout the perioperative period, for all sorts of indications
- There's no blanket rule, but clopidogrel and aspirin are generally stopped 5-7 days before elective surgery
- DOACs are stopped between 2-5 days before surgery depending on the drug and the patient's renal function
If you're struggling for words in an exam, 'An individualised assessment of thrombotic risk in discussion with haematology' is never a bad shout.
What surgical techniques can help reduce blood loss?
- Laparoscopic surgery
- Robot assisted surgery
- Endovascular technique
- Topical haemostatic agents
You often also get marks for 'meticulous technique' and 'senior surgeon' so they're worth mentioning.
Few would disagree that bleeding is primarily a surgical issue, but you as the anaesthetist can certainly do a lot to help out.
How can the anaesthetist reduce intraoperative blood loss?
- Regional anaesthesia (particularly in orthopaedics and obstetrics)
- Tranexamic acid
- Maintain haemostasis with particular attention to:
- Hypothermia (core temperature above 35°C)
- Acidosis (pH >7.2)
- Hypocalcaemia (aim >1mmol/litre ionised calcium)
- Optimise blood pressure - enough to perfuse the brain, but not too much to worsen bleeding*
- Patient positioning - ensuring good venous drainage from the operative site will reduce avoidable bleeding
- Smooth and calm team leadership, coordinating cell salvage and transfusion efforts
*If you're asked for specific examples of manipulating blood pressure, you can mention hypotensive anaesthesia for ENT or Spinal surgery, and reduction of central venous pressure for liver resection.
When to transfuse?
So you've pre-optimised your patient, identified that they're at risk of bleeding, ensured the best consultant surgeon is doing their best laparoscopic work, given tranexamic acid and running a beautiful blood pressure of 92/68, but the patient is still haemorrhaging.
Time to start transfusing.
Now we tend to have two distinct categories of patient that we end up transfusing:
- Those who are bleeding a small amount, continually
- Those who are bleeing lots, and right now
It's important to know which category your patient is in, because the guidance is different.
A small amount continually
Examples of this include your medical bleeders - (peptic ulcers and varices) - and your post-operative bleeders - (haematomas, abdominal drains etc)
Lots of big important studies have come to the conclusion that a restrictive transfusion threshold is safe and sensible - so we end up using the arbitrary number of 70 g/L most of the time.
- Only give what you need, don't reflexively give two units of red cells at a time just because 'that's what we normally do'
- Aim for 70 in normal patients, and 80-90 in those with cardiovascular disease
If they're symptomatic, and have lost a lot of blood, but have an Hb slightly above the transfusion threshold, then transfuse them now - it's likely their Hb will drop below 70 by 3am.
Lots and right now
Ignore their haemoglobin, and treat the patient using your clinical acumen and - more importantly - common sense.
- If a patient loses two litres of blood in ten minutes and you test their haemoglobin - it will still be normal, because the remaining blood hasn't had a chance to dilute yet
- If the patient is clearly in a state of cardiovascular compromise as a result of significant and active blood loss, (tachycardic, poor sats trace, hypotension) then you need to activate the major haemorrhage protocol, because you don't know how long this bleeding is going to continue
- By all means give a bolus of crystalloid while waiting for blood, but do not delay transfusion
It is considerably more preferable to have activated the MHP and not need it, than vice versa.
After all, the MHP has an unused item returns policy that would make ASOS proud.
The main players
If we're honest, much of the time we end up giving a couple of units of red cells and calling it a day.
But if you're managing a 'proper' major haemorrhage, you ideally want to be giving the following in a 1:1:1:1 ratio where possible
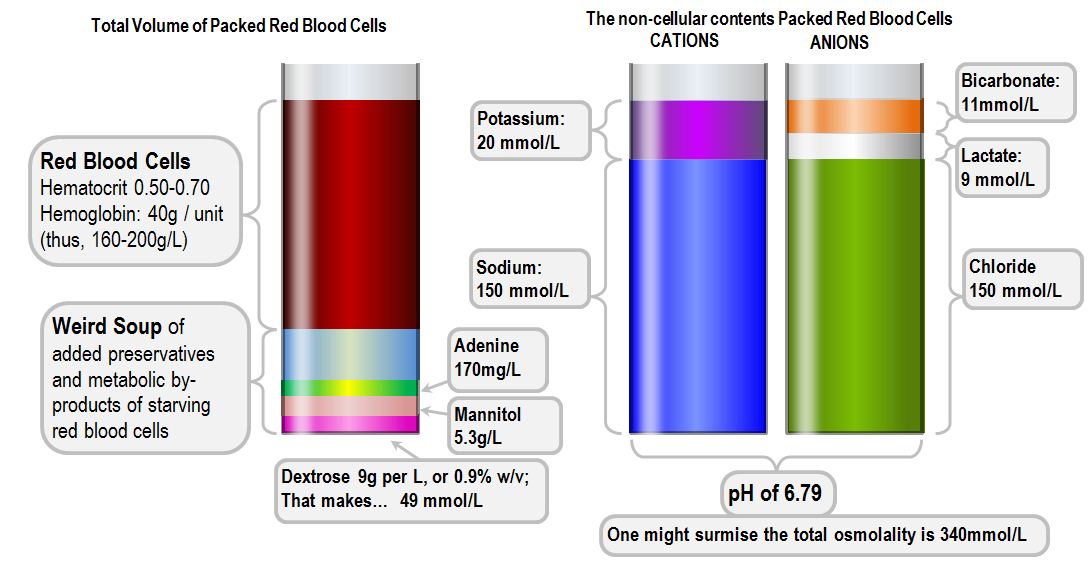
Packed Red Cells
- Stored at 4°C to inhibit bacterial growth and reduce RBC energy consumption
- Stored in one of two dedicated solutions - SAGM or CPDA1
SAGM - Saline, adenine, glucose and mannitol
CPDA1 - citrate, phosphate, dextrose and adenine
- Citrate chelates calcium and prevents clotting
- Phosphate is a buffer
Packed red cells can be stored for up to 35 days, however there will be gradual deterioration over time:
- Only 70% of the red cells will survive that long
- Haemolysis
- Hyperkalaemia
- Acidosis
- Loss of 2,3-DPG
Group O is the universal donor, and Group AB is the universal recipient, all others need matching up.
Fresh Frozen Plasma
- Plasma from one donor
- 150ml bag full of clotting factors, albumin and gamma globulin
- Stored frozen at -20°C and needs to be used immediately once thawed
- Needs to be ABO compatible*
- Dose is 10-15ml/kg (four packs for an average adult)
When to give:
- INR >1.5 and requiring some sort of invasive procedure or actively bleeding
- Pre-emptively as part of major haemorrhage protocol
*Remember that the donor/recipient system is the other way round for FFP, because the donor plasma contains the antibodies rather than the antigens so AB FFP is the universal donor.
Cryoprecipitate
- FFP is thawed out slowly, and the precipitate is skimmed off
- Stored at –30oC for up to a year
- Cryo contains higher levels of factor 8, VWF and Fibrinogen than FFP
- One unit is about 30ml
- Needs to be ABO compatible
When to give:
- If fibrinogen less than 0.8 g/L
- If fibrinogen <1.0 and actively bleeding
- Pre-emptively as part of massive transfusion protocol
- Disseminated intravascular coagulation
- Hereditary hypofibrinogenemia
- Haemophilia
- Von Willebrand disease
Ten units of cryo will raise fibrinogen by 1 g/L
Platelets
- See below
You might also consider some PCC.
Prothrombin complex concentrate
- e.g. Beriplex
- Contains clotting factors 2, 7, 9 and 10
- Contains protein C and S
When to give:
- For immediate reversal of raised INR in bleeding patient on warfarin
- Warfarin overdose with risk of life threatening bleeding
- When all else fails (chat to Haematology first)
All platelets are not equal
There are two types of platelets - random donor platelet concentrates from units of whole blood, and apheresis platelets from a single donor, harvested via the unimaginatively titled process of plateletpheresis.
- RDPs contain 50x109 platelets in around 50ml of plasma
- Apheresis platelets come as a 250ml (ish) bag containing 300-400x109 platelets
Platelets have ABO and Rhesus typing, so ideally compatible platelets should be given, however in reality this is rarely of clinical importance and you often don't have much choice anyway.
What are the indications for prophylactic platelet transfusion?
In a patient that is not bleeding
- Platelets less than 10
- Platelets less than 20 if septic or coagulopathic
In a patient who is bleeding, or having a procedure done
- Platelets less than 50
- Platelets less than 80 if needing neuro or eye surgery
- Recent antiplatelet administration or known platelet dysfunction
Please warm them up
The trauma triad of death refers to the three things that make bad bleeding much worse:
- Hypothermia
- Acidosis
- Coagulopathy
So now lets look at our friendly bag of packed red cells...

- Packed red cells are stored at 4°C, in citrate, with a pH of 6.5 and a lactate of 25mmol/litre
Here's what happens if you rapidly infuse a cold bag of red cells
- It cools the patient's core temperature down by about 0.25°C
- These cold red cells have very poor oxygen carrying capacity, meaning they're not a whole lot better than crystalloid
- The citrate will bind the patient's ionised calcium leading to hypocalcaemia and coagulopathy (this can happen with warm cells too, but it's worse if they're cold)
- Oh, and the patient is probably already churning out a fair whack of lactic acid if they're bleeding badly enough to need rapid transfusion, and cooling them down makes this problem worse as well
- Even more oh, you'll remember from your oxygen haemoglobin dissociation curve that hypothermia drives the curve to the left, making it less good at delivery oxygen to already hypoxic tissues, so you've made everything so much worse and you should probably just stop now
Warm the patient, and warm the blood.
What else is going on in that bag?
- Hyperkalaemia - increase in potassium by around 5-10 mmol per unit, however this doesn't usually present a clinical problem as the Na/K ATPases crank this all back into the red cells once they're warmed up inside the patient
- Acid and Alkali - A unit of red blood cells contains up to 2mmol of acid, (citric acid from the anticoagulant and lactic acid from the red cells). Citrate is metabolised to bicarbonate in the liver, which can cause a metabolic acidosis if lots of red cells are transfused
What else can we do?
Cell salvage
It makes logical sense to try and hoover up the patient's own spilt blood and put it back from whence it came.
Clearly it needs a bit of cleaning and whatnot first, but in essence you can often avoid significant blood transfusion by salvaging as many red cells as possible and returning them to the patient.
What is cell salvage?
- If you're anticipating blood loss greater than 1000ml, consider using it
- Uses a double lumen suction catheter to collect blood from surgical site
- It then cleans and centrifuges the blood to separate out the components
- The red blood cells are stored ready for infusion back into the patient
Unsurprisingly, it is generally recommended to use tranexamic acid if you've got cell salvage under way.
Antifibrinolytic drugs
Don't forget fibrinolysis in all the chaos of managing major haemorrhage. All the while we're fighting to get a clot to form, our little friend plasmin is sat merrily chewing away at our carefully crafted fibrin mesh, and undoing all of our clotting factors' hard work.
A quick bash on the head with some tranexamic acid and plasmin will leave you alone for a little while, allowing the blood to form a stable clot.
Can you name any antifibrinolytic drugs?
- Tranexamic Acid
- ε-aminocaproic acid
These are lysine analogues that inhibit fibrinolysis by targeting plasminogen, preventing the production of active plasmin.
Go check out the CRASH-2 trial at some point.
TEG and ROTEM
These point of care tests have been game changers for haemorrhage management.
Check out our full post on TEG and ROTEM here
The main points:
- Point of care testing reduces transfusion requirements
- They're particularly good for assessing fibrinogen levels
What could possibly go wrong?
If you do go ahead and decide to inject bits of someone else's blood into a patient's veins, then you need to be aware of the complications that can occur.
There are a variety of reactions that vary in both their immediacy and severity, either as a reaction to the blood products themselves or the sheer volume that has been infused.
Immediate reactions
These can be immune or non-immune.
Immune:
Acute haemolytic transfusion reaction
- Antibodies in patient's plasma react to surface antigens on the donor red blood cells leading to RBC destruction and haemolysis
- Usually there is previous exposure, either transfusion or placental transfer
- Mostly due to ABO and Rhesus D incompatibility
- There are over 250 red cell antigens that could cause this
- The most serious are anti-A and anti-B
- IgM or IgG are usual culprits
Signs and symptoms:
- Headache
- Chest pain
- Flank pain
- Fever
- Flushing
- Chills and rigors
- Nausea and vomiting
- Urticaria
- Dyspnoea
- Hypotension
- Haemoglobinuria
- Disseminated intravascular coagulopathy
Clearly some of these are harder to spot in an anaesthetisted patient, so a sudden drop in blood pressure may be your first indication.
Non-haemolytic febrile reactions
- Very common
- Usually nothing to worry about
- Caused by donor leucocyte antigens reacting to patient plasma antibodies
- They form a leucocyte antigen-antibody complex
- This complex binds complement
- This results in release of Interleukin-1, IL-6 and TNFα
- Usually kicks in during or a few hours after the transfusion
- Severity is related to speed of transfusion and quantity of leucocytes
Reactions are much less common than they used to be, as pretty much all transfusions are leucodepleted these days.
A direct antiglobulin test will tell you if it's a haemolytic reaction or not.
- This will be negative in the case of a febrile reaction as there won't be any plasma antibodies attached to the donor red cells
Anaphylaxis and urticaria
Allergic reactions are weirdly common in blood transfusions.
- Donor plasma contains foreign proteins which cause mild IgE mediated reactions
- Usually this manifests as itching or a rash, without substantial cardiorespiratory compromise
- Thankfully anaphylaxis is rare
The best thing to do is usually to stop the transfusion, give an antihistamine such as chlorphenamine, and then if the patient is well in thirty minutes or so, the transfusion can slowly continue.
If they react again then the transfusion should be abandoned.
- Patients with a hereditary IgA deficiency and anti-IgA antibodies are at particular risk of anaphylaxis
- These patients should receive washed PRC transfusions, as these have any residual plasma removed
Transfusion Reaction Associated Lung Injury
This is classic exam fodder so it pays to know it in a decent amount of detail.
TRALI has two mechanisms
- immune
- non-immune
Immune is due to white cell antibodies in the donor plasma, targeting HLA (Human leucocyte antigens) and HNA (Human neutrophil alloantigens) in the recipient.
- In around 40% of cases, no antibodies are found, and so it's thought that a reaction is triggered by release of membrane lipids from the donor cells
- In either case the main culprit is the neutrophil granulocyte
These get stuck in the pulmonary microvessels and then release free radicals and enzymes causing destruction of the endothelium
- 70% of patients end up needing mechanical ventilation
- 6–9% of cases are fatal
A definitive diagnosis requires antibody detection
- TRALI can be in response to whole blood, PRC, Platelets or FFP
- Thought to be between 1:1000 to 1:5000 transfusions
Non-immune:
Complications of massive transfusion
- Hypothermia
- Hypocalcaemia and citrate toxicity
- Air embolism
- Hypomagnesaemia
- Acidosis
- Hyperkalaemia
Traumatic haemolysis
- Caused by physical trauma to red cells, rather than an immune response
- Can cause clinically detectable jaundice
Infection and sepsis
These are thankfully rare nowadays as a result of vigilant screening processes and leucodepletion of blood products, but they're still possible, and still examinable.
As with any question about infections, break it down into the following categories:
Bacteria
- Uncommon but associated with high mortality fulminant sepsis
- Can occur due to contamination from venepuncture, or from a bacteraemic donor
- Packed red cells are stored at 4°C, making infection with gram-negatives such as Yersinia and Pseudomonas more likely
- Gram-positives such as S. Aureus and Bacillus don't enjoy the cold and are more commonly associated with platelet contamination
- Don't transfuse a bag of blood that looks darker than usual or has gas bubbles
Viruses
- Much lower infection rate since better screening of donors
- Donor blood is screened for Hep B, Hep C, HIV 1 and 2, CMV and syphilis
- Infection is still possible if infection not detected by screening due to timing of testing and inoculation
Risk of infection from a single unit of packed red cells:
- Hep B = 1/100 000
- Hep C = 1/1 000 000
- HIV = 1/4 000 000
- Hep A = essentially zero
Prions
- Theoretically possible to transfer CJD prion disease through blood transfusion
- To avoid this, blood is leucodepleted as standard, and people who received transfusions before 1980 are not allowed to donate blood
Terrifyingly there is currently no test or treatment for CJD, so that's fun.
Transfusion associated circulatory overload
- Essentially the same problem as giving too much fluid to someone with heart failure
- Patient can develop pulmonary oedema and acute heart failure
- Often difficult to distinguish from TRALI
Delayed reactions
Much like their acute counterparts, delayed reactions can be categorised into immune and non-immune.
Delayed immune reactions include:
- Delayed haemolytic transfusion reaction
- Graft vs Host disease
- Alloimmunisation
- Transfusion related immunosuppression
The only real non-immune delayed reaction to worry about it iron overload in patients that have chronic transfusions
Delayed haemolytic transfusion reactions
These are usually due to incompatibility between the more minor blood group subtypes such as Kidd and Rhesus.
- The antibodies can get missed during the antibody screening process because of their very low levels, but these then increase dramatically upon exposure to the antigen - this is called an anamnestic response
- This type of reaction doesn't trigger the complement system, meaning there is extravascular haemolysis via IgG, rather than intravascular
Delayed haemolytic transfusion reactions can be confirmed in three ways:
- New unconjugated hyperbilirubinaemia causing jaundice
- Unexpected sudden drop in haematocrit
- Positive direct antiglobulin test
Graft vs Host disease
- It's very rare, largely due to leucodepletion
- 90% of cases are fatal
- Donor T cells attack recipient host tissues
Features:
- Maculopapular rash
- Abdominal pain
- Diarrhoea
- Deranged liver function
- Pancytopenia
Irradiating blood destroys or inactivates donor leucocytes, and generally avoids this problem.
Alloimmunisation
- Previous exposure and sensitisation leads to antibody production on repeat exposure
- Similar to the issues seen when a rhesus-negative mother has a rhesus positive baby
Transfusion related immunosuppression
- Patients that receive blood often demonstrate a period of immunosuppression afterwards
- This is probably due to the 'distraction' of the patient's white cells by the invading visitors
What to do if a patient has a reaction
- Recheck the right blood is being transfused
- Rule out anaphylaxis as a priority
- Stop or slow the transfusion - restart slowly if symptoms settle
- Give paracetamol
- Consider chlorphenamine
- If severe reaction then stop and resuscitate with fluids, pressors, ventilatory support and adrenaline as required
Reporting reactions
The Blood Safety and Quality Regulations 2005 require that all serious adverse
transfusion events/reactions must be reported to SHOT; a number of these will also
be reported to the MHRA. This is done via the hospital Blood Bank.
Mild febrile reactions do not need reporting.
If a patient has a reaction to a blood product then you need to do the following (after resuscitating them first):
- Return suspect blood product unit and giving set to blood bank
- Return all units transfused in the previous 24 hours to blood bank
- Send a repeat group and save
- Send a clotting and full blood count
- Send biochemistry, LFTs, haptoglobin
- Send blood cultures
The Serious Hazards of Transfusion Scheme (SHOT) should be informed, you can check out their website here

Useful Tweets
Hemolytic Anemia
— Aaron Goodman - “Papa Heme” (@AaronGoodman33) December 27, 2022
A roadmap to diagnosing all causes of hemolytic anemia!
Simplified:
Prove hemolysis = ⬆️Retic (RPI), i. bili, LDH, ⬇️hapto
Check D. Coombs!
Positive = immune mediated (warm vs cold vs post transfusion or drug)
Negative use chart below!https://t.co/3CjADAypcD pic.twitter.com/k2Aamoxdad
References and Further Reading


Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.

