Ventilator Associated Pneumonia
Take home messages
- VAP is the most abundant ITU infection that the patient didn't bring in with them
- Nobody can agree on what actually constitutes a VAP
- Everyone agrees it makes everything worse
Podcast episode
What is it?
The broadly accepted definition is 'any pneumonia occurring after 48 hours of invasive ventilation'.
- VAP is also therefore a type of HAP
- You have to be intubated - if you get pneumonia after 48 hours of NIV it's still just a HAP
- If it's less than 96 hours it's early onset and after that it's late onset
What are the consequences of VAP?
- Increased mortality and morbidity
- Increased days on the ventilator
- Increased ITU stay
So why does it happen?
"But how can you aspirate if you have a cuffed endotracheal tube properly inflated in the right place?"
Good question.
The thing they don't advertise in the 'how great intubation is' class is the fact that the low pressure, high volume cuffs that we enjoy using - because they cause less tracheal mucosal damage - are also more prone to forming little folds and gulleys in the cuff material (because there's more of it) when inflated, forming tiny little channels that secretions can trickle down over long periods of time.
Hence the term 'microaspiration'
To make things worse, the patient has usually had all their 'good' bacteria obliterated by the tazocin shower at the front door to critical care, and so rapidly recolonises their oropharynx with nasty gram negative aerobes, making any aspiration far more likely to grow something unpleasant.
Oh, and they're usually critically ill and therefore severely immunosuppressed.
How can we modify the tube?
Surely if the tube is the cause of all our worries, we can just change something to stop this whole problem from happening?
- The ferocious high pressure, low volume cuffs often favoured by HEMS et al. are far less prone to microaspiration, but then again - mucosal ischaemia isn't exactly good either
- There are some new tubes with tapered cuffs made of ultra thin polyurethane which appear to be the best at preventing microaspirations
- Some tubes also have an antimicrobial or silver coating to help prevent biofilm formation, but these are more expensive and less widely available
Furthermore, being intubated generally prevents coughing and reduces the patient's airway reflexes, meaning the evolution-proven mechanism of natural pulmonary clearance is completely obliterated, and microbes are given much more opportunity to settle and set up shop.
As soon as a biofilm forms, we're in trouble, because this is relatively immune to antibiotics and acts as a source of continuous re-infection as the ventilator ambivalently shoves bits of it into the smaller airways twelve to thirty times a minute.
What factors increase risk of ventilator associated pneumonia?
- Longer duration of ventilation
- Supine position
- Enteral feeding via NG tube*
- Comorbidities - renal failure, anaemia, malnutrition, chronic lung disease
- Micro or macroaspiration
- Age >55
- Polytrauma
- Muscle relaxant use
- Thoracic and upper abdominal surgery
- Multiple courses of antibiotics
- ARDS
- Reintubation
- Frequent ventilator circuit changes
*There's some evidence to suggest that feeding beyond the pylorus results in less regurgitation and aspiration, however this still needs work.
How bad is VAP?
Well you wouldn't exactly prescribe it.
Your patient has come into hospital with whatever ailment has been deemed sufficiently terrible that they're now on a ventilator on intensive care, and now they've got another terrible ailment that is sufficiently deadly in its own right, so yeah - not good.
Interestingly, the earlier you get a VAP, the better you tend to fare, presumably because these bugs are easier to kill, antibiotic-sensitive community bacteria (think Haemophilus and Streptococcus), while the super-resistant hospital ones (Pseudomonas etc) generally take a little longer to emerge.
A VAP after 5 days is usually bad news
It is estimated that between 10 and 30 ish % of intubated patients will develop a VAP, and once they've got it, mortality sits between 25 and 50%.
Diagnosis
"Diagnosing pneumonia is absolute bread and butter med school stuff, surely it can't be that hard?"
Well hold on now just a minute - It's not that simple.
While pneumonia is a generally straightforward diagnosis, nailing down VAP as the exact cause of your patient's new fever/oxygen requirement/xray findings when they've got another critical illness churning away in the background isn't such a walk in the park.
What conditions may mimic VAP?
- Pulmonary oedema
- Bronchitis
- Pulmonary embolism
- Contusion
- Infarction
- ARDS
It's certainly nuanced enough that there isn't yet a definitive set of diagnostic criteria, but a lot of people choose the CPIS score as a solid benchmark.
How is VAP diagnosed?
Via a combination of clinical, radiological and microbiological criteria, using clinical suspicion and a sprinkle of guesswork.
Clinical features
- Purulent secretions
- Fever
- Raised white cell count
- Crackles or fremitus on examination
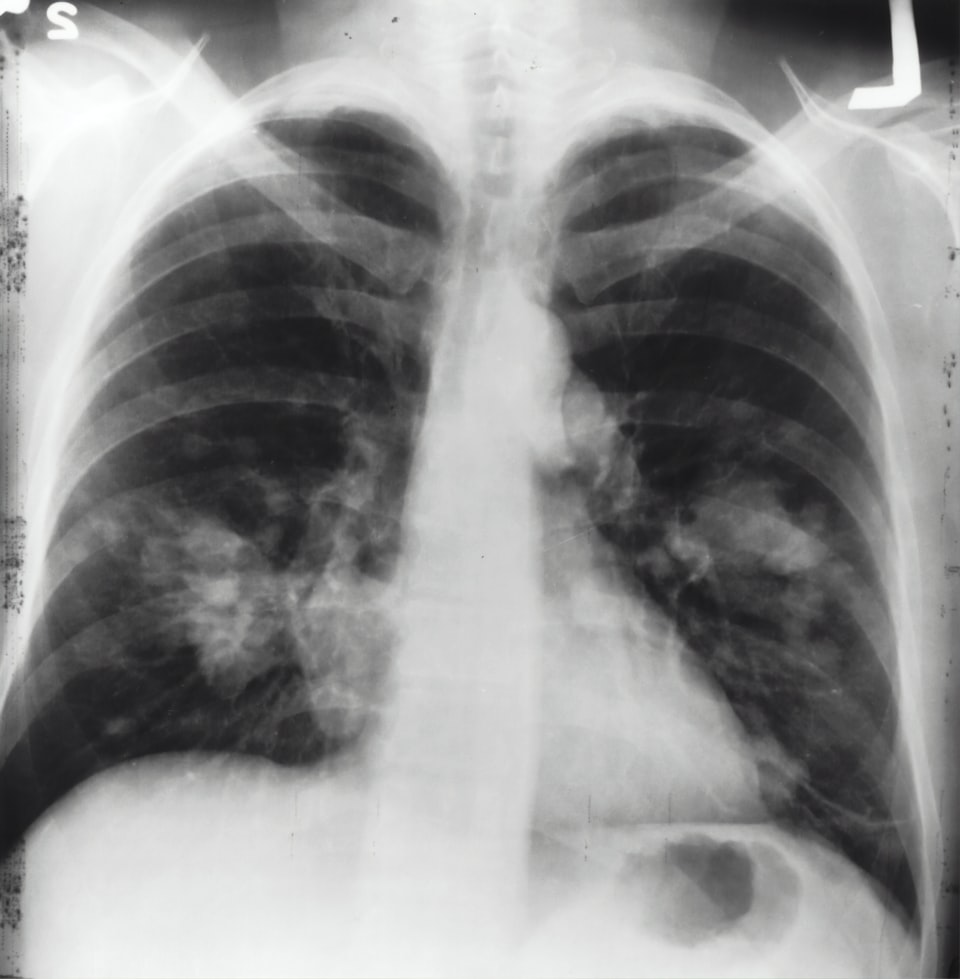
Radiological
- Infiltrates on chest xray (enormously non-specific on ITU)
- Individual air bronchograms (specific but not sensitive)
- Fissure abutment (specific but not sensitive)
Microbiological
- Non-directed alveolar lavage
- Bronchoscopic alveolar lavage (104 colony forming units per millilitre)
- Tracheal aspirates (more likely to be contaminated with upper airway bacteria)
On their own, each of these criteria are rather useless in terms of sensitivity and specificity, but together they're a fairly robust way of diagnosing VAP.
Developed by Pugin et al. to aid diagnosis using clinical parameters
Each of the following is awarded points on a scale of 0-2:
- White cell count
- Temperature
- Secretions
- Sputum culture
- Pao2/FIo2 ratio
- Chest Xray
A score of 6 out of 12 is considered diagnostic for VAP, however the xray and tracheal secretions bits mean there is substantial observer-variability of results.
The Johannson Criteria, however, are probably closer to your usual diagnostic process at 3am on ITU.
The Johannson Criteria
- New or worsening xray infiltrates
Plus at least two of:
- White cells
- Temperature >38°C
- Purulent secretions
How do I treat it?
Pretty much like any other pneumonia, it's just harder because usually it's a nastier bug doing the dirty work.
What antibiotics should I use?
- As always, refer to your local microbiology guidelines
- These should be informed by patterns of local resistance
- Take cultures but don't wait to start antibiotics
- If you're starting them on antibiotics for the first time, then something like co-amoxiclav or ceftriaxone is sensible
- But let's be honest, they're all getting Tazocin +/- meropenem if they're really sick
- Gentamicin is rarlely a bad idea, to cover the multi drug resistant strains
- If you're suspecting MRSA, consider linezolid
- If you're suspecting Legionella, consider something ending in -floxacin
7 days appears to be the agreed standard course duration - however clearly this is going to vary between patients and you should assess daily for response.
Should I try nebulised antibiotics?
Well it's not a terrible idea.
The idea
- The drugs that work for VAP such as gentamicin generally have poor penetration of lung tissue, so nebulising them directly onto the biofilm and affected lung tissue seems sensible
- You can achieve a very high local concentration with minimal systemic side effects, and potentially avoid resistance
- It also won't affect the gut microbiome to nearly the same degree
However
- Can trigger bronchospasm and block HME filters
- Hard to figure out what doses are safe and needed
- Needs a special nebuliser
- It's tricky to get the droplets at the right size - often they're too big and just collect on the tube and trachea
I'd probably talk to micro first.
How can I prevent it?
Clearly the obvious answer is 'avoid intubation' which we generally try to do anyway.
Along with that we have the strongly-evidenced 'minimise duration of ventilation' package which includes:
- Use of NIV where possible
- Regular sedation holds and extubate as early as possible (but safely)
- Avoid re-intubation
- Early mobilisation, physiotherapy and lots of coughing
Beyond that we have lots of little interventions that don't make a whole lot of difference on their own, but if done properly in tandem they can make a big difference.
When asked about VAP in any exam context, try and mention 'care bundles' at some point in your answer.
What on earth is a care bundle?
Listen up, because this gets examined a whole lot in the Final FRCA exam.
High Impact Intervention No 5 - Care bundle for ventilated patients
You can find it here
The original parameters were the following four:
- Head of bed elevation to 30-40°
- Sedation holds
- DVT prophylaxis
- Gastric ulcer prophylaxis*
Sedation holds reduce time spent intubated and also allows the patient to cough (even while intubated), both of which substantially reduce the incidence of VAP.
Added extras include:
- Humidified circuits
- Regular tubing replacement
- Regular suctioning (particularly subglottic)
- Tracheal tube cuff pressure monitoring (20-30 cm H2O)
- Oral hygiene
*Interestingly, PPIs and stress ulcer prophylaxis in general seems to increase the risk of VAP, presumably by making the stomach more hospitable for bacteria.
NG tubes
We like NG tubes on intensive care, but if you consider the fact that they act as what is essentially a fireman's pole for bacteria to track from nasopharynx to glottis, while simultaneously splinting the upper and lower oesophageal sphincters open to facilitate a cheeky bit of reflux, it's easy to see how they increase the rates of VAP.
If you need to use an NG tube:
- Consider post-pyloric feeding via NJ or even ND if that's all you can manage
- Consider selective digestive decontamination (contentious)
- Reduce stress ulcer propylaxis use (which isn't generally needed once enteral feeding is established anyway)
Selective digestive decontamination
There is no agreement here as to whether this is a good plan or not.
- Some people think that using chlorhexidine or antibiotics to decontaminate the oropharynx is a sensible idea
- Logically it makes sense that sterile secretions are less terrible than those laden with pathogens
- Clearly however this can make resistance worse
- Currently the American Thoracic Society advise that 'routine use is not recommended' at least not until there's stronger data
Useful Tweets and Resources
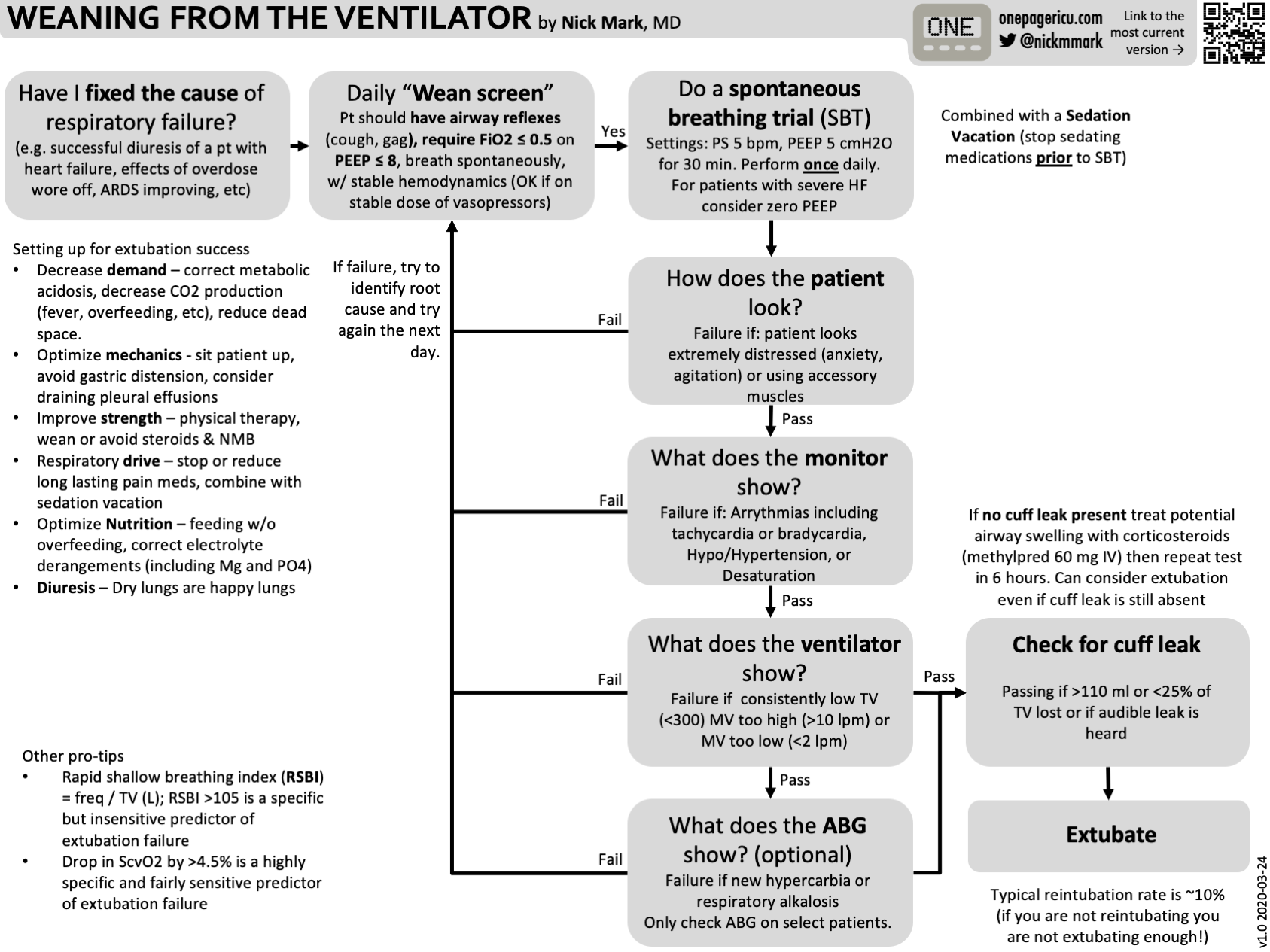
As always Nick Mark MD over at onepagericu.com has devised a brilliant infographic on weaning patients from the ventilator to help reduce time spent intubated and the incidence of VAP
Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Literature Review
— Critical Care Reviews (@CritCareReviews) January 27, 2024
CCR Journal Watchhttps://t.co/Sp06oA6IDG
Get the latest critical care literature every weekend via the CCR Newsletter - subscribe at https://t.co/nitMzacLrj pic.twitter.com/FPxYsKHZRx
References and Further Reading


Primary FRCA Toolkit
While this subject is largely the remit of the Final FRCA examination, up to 20% of the exam can cover Primary material, so don't get caught out!
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.

