The Clark Electrode

Take home messages
- The Clark electrode measures oxygen tension in a fluid sample
- It is a polarographic electrode, meaning it measures current rather than voltage
- It thus requires a 0.6V power supply
Why you need to know this
The Clark electrode is prime exam fodder at both primary and final levels, and it's one of those topics where, if you take the time to understand it, you can win a load of easy marks on exam day.
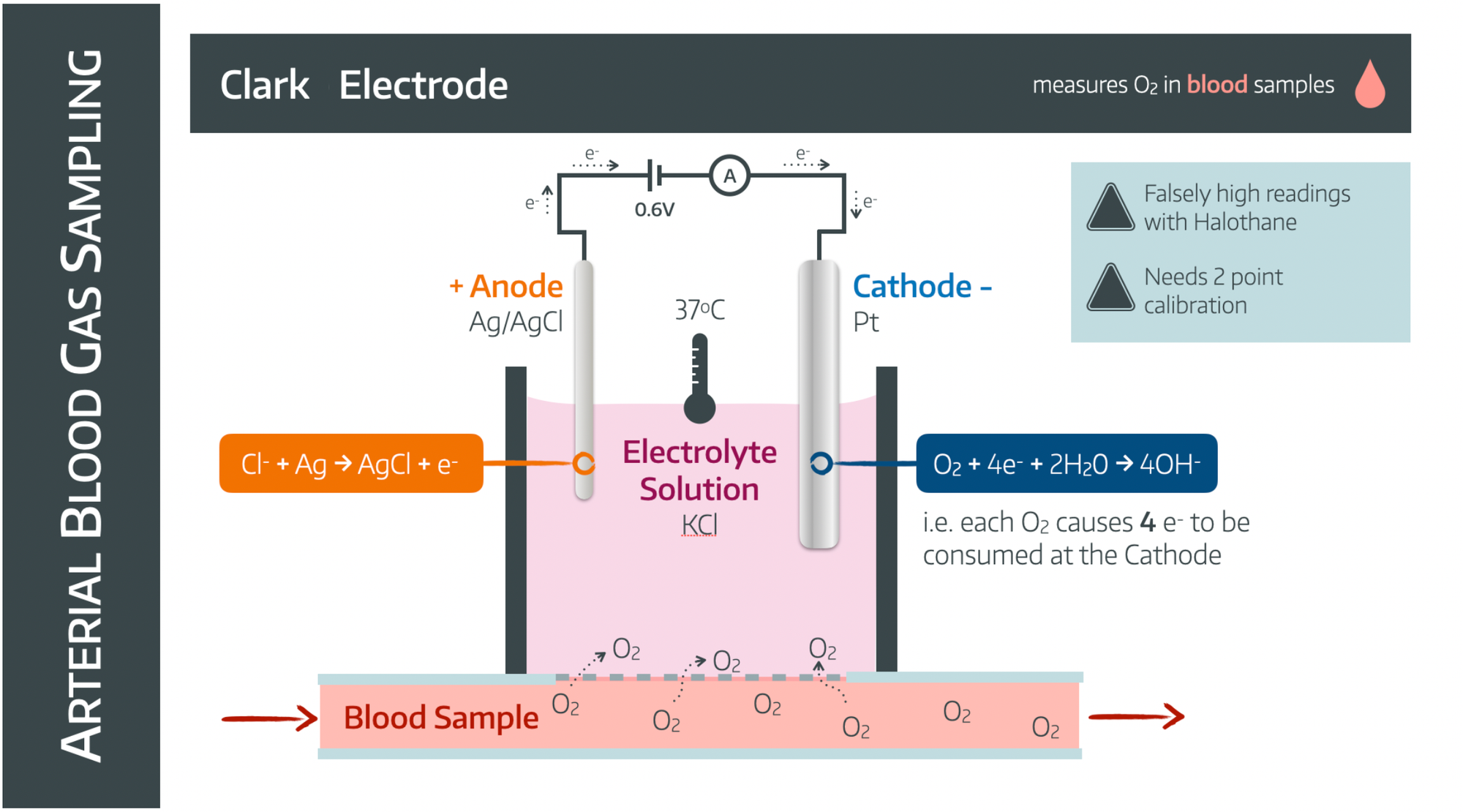
How it works
You can choose to simply memorise the above diagram if you'd prefer (or the exam is tomorrow), or we can actually understand it, and never have to memorise it again.
Let's build it up from the basics, so it actually makes sense.
Start with a reaction
We want to measure the oxygen tension, using electrical equipment, so we need some sort of reaction that turns 'change in partial pressure of oxygen' to 'change in electricity'.
- This can be a change in current or voltage
- This means we need a reaction that either causes electrons to flow, or to build up on one side of a circuit
Either way, we need an equation that contains oxygen, and a change in electrons.
In this equation, we can see that we start with oxygen and electrons on the left, neither of which are present on the right.
Assuming a steady unlimited supply of electrons and water, the rate of this reaction will depend entirely on the partial pressure of oxygen.
Perfect.
Where are we going to get our electrons?
Some sort of electrode sounds like a sensible starting point, and we're going to use platinum for the following reasons:
- It is a metal, meaning it will gladly supply electrons, if encouraged to do so*
- It's a very effective catalyst of the above reaction, making the whole process more efficient and speedy
- It's resistant to reacting with stuff itself, so it's durable and doesn't mess with the numbers
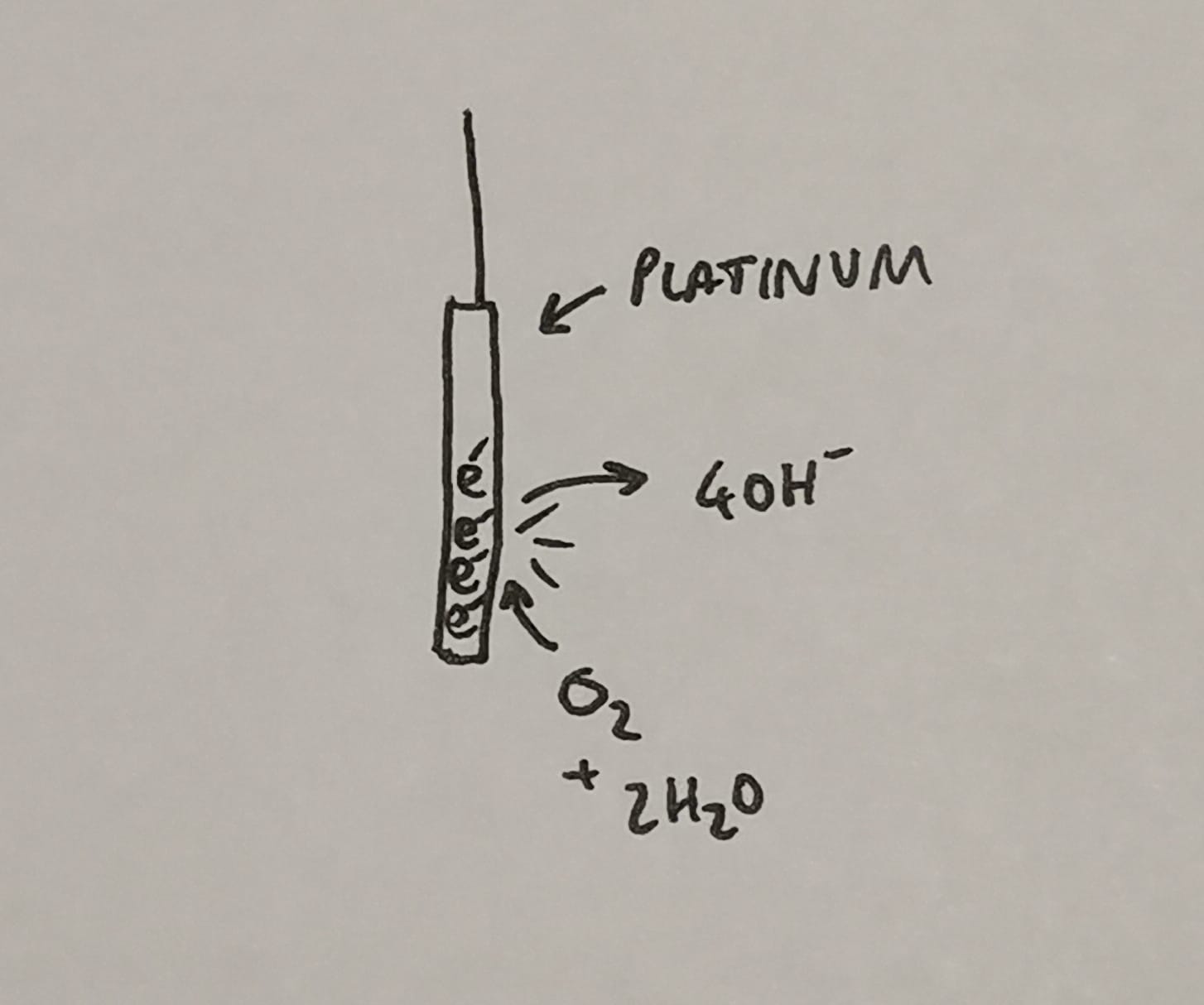
*This brings us to our next issue.
How do we encourage it to give up electrons?
Well, we could shove so many electrons into the electrode that it doesn't know what to do with them, and to do this, we need a voltage, from a power supply.
What does the voltage do?
It is needed to polarise the electrodes, and this achieves three things:
- It drives the reduction of oxygen at the platinum cathode, ensuring a steady supply of electrons to push the equation from left to right
- By forcing this process forwards, it also ensures there is a linear response (in terms of current change) with changes in oxygen tension
- It also helps drown out noise from other interfering redox reactions
Where are we getting the electrons from?
Ah. Good point.
It's all very well having a power source driving electrons down into our platinum electrode, but we need to source these electrons from somewhere.
It would be simply magical if we could find a second reaction that doesn't involve oxygen (and thus won't mess with our measurements) that will churn out a steady supply of electrons on demand.
Enter the silver/silver chloride electrode.
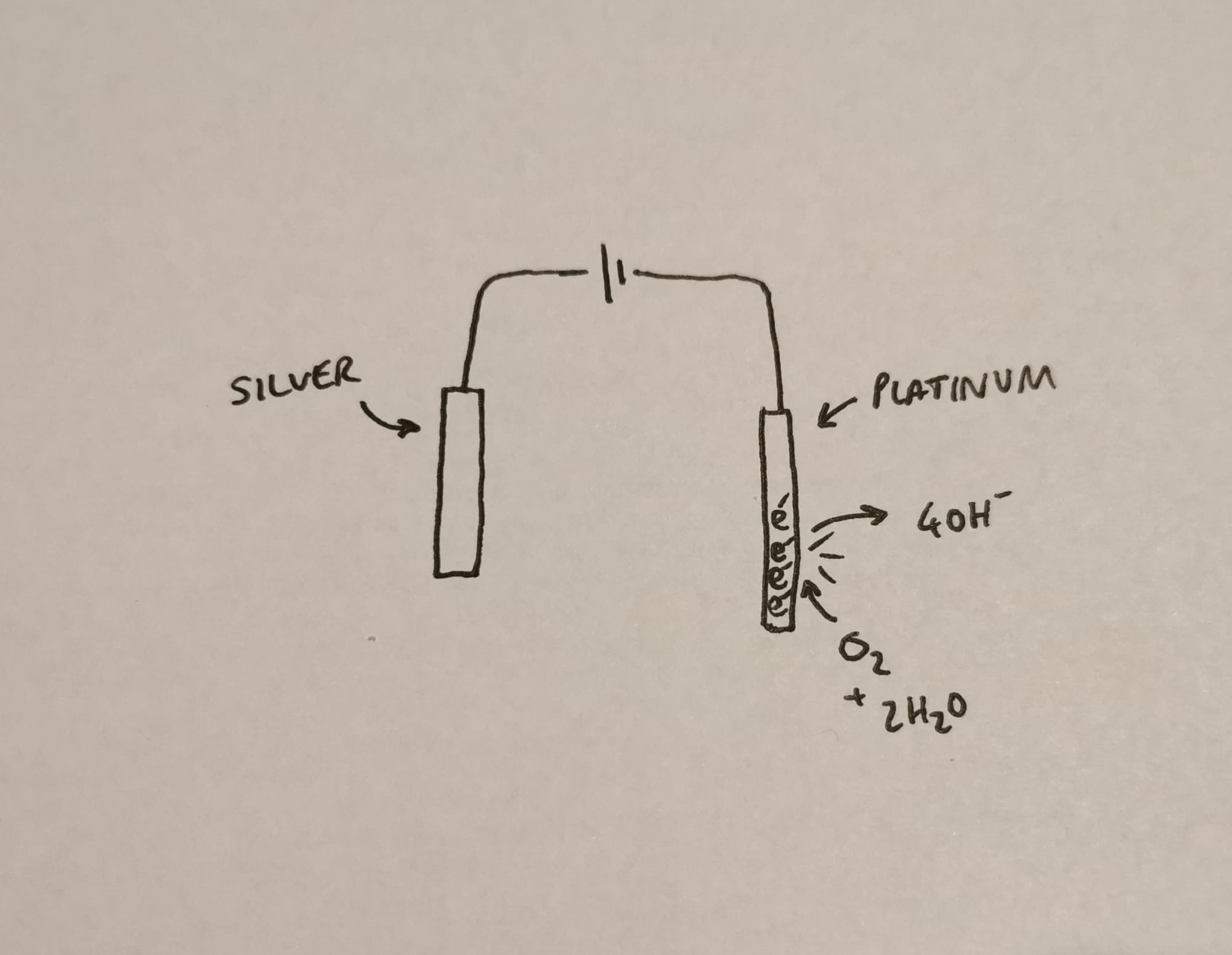
If you stick a block of silver on the other end of your power supply and start ripping electrons out of it, it's actually really rather a good sport about the whole thing
As long as you have enough silver, it'll keep merrily pouring out electrons as long as there's enough voltage to drink them up.
Of course this leaves us with a rather pertinent issue of the increasing quantity of silver ions left lying around, so we'll throw in some chloride ions to mop them up shall we?
This source of chloride ions is found in the electrolyte solution (KCl) in which our electrodes are immersed.
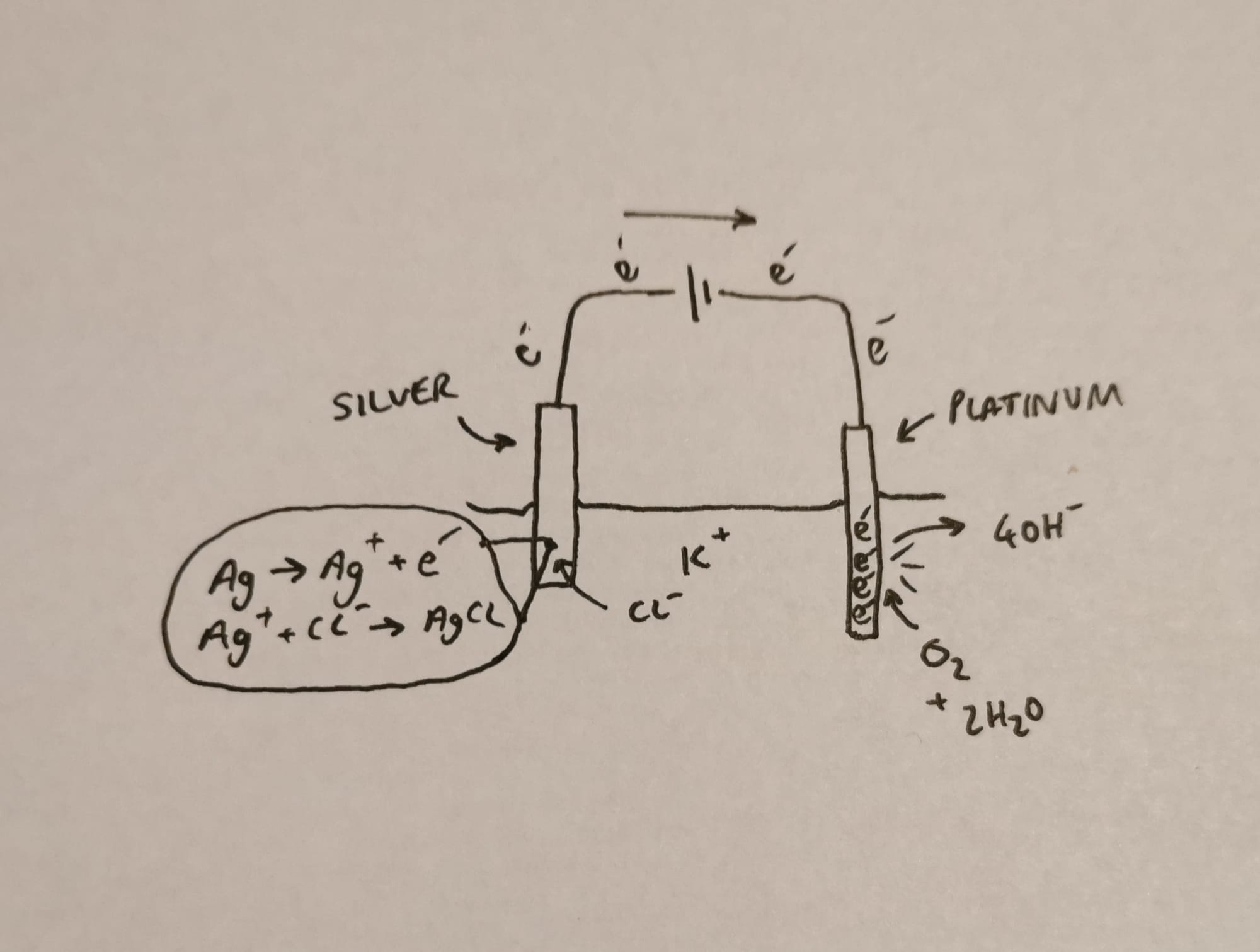
There.
Much better.
Hang on, aren't we slowly consuming silver?
Yes, and this is why it's called a silver/silver chloride electrode, because the silver gradually gets consumed as it is converted into silver chloride.
- You will eventually need to replace the silver electrode
- This is one of the disadvantages you can mention in the exam
Now we need to let oxygen in and start measuring
Fabulous, lets:
- Wrap everything up in an oxygen-permeable membrane, made of polytetrafluoroethylene or Teflon
- Throw in an ammeter as well, to measure the current
- Calibrate to a few known samples so we know what currents we're expecting
- Let our blood sample pass across the other side of the membrane
Now we can package this whole contraption into a neat pokey stick that can be dipped into a blood sample.
Behold, a machine that will generate and measure a current that will change linearly with changes in oxygen tension, for all clinically important PO2 values.
Beautiful.
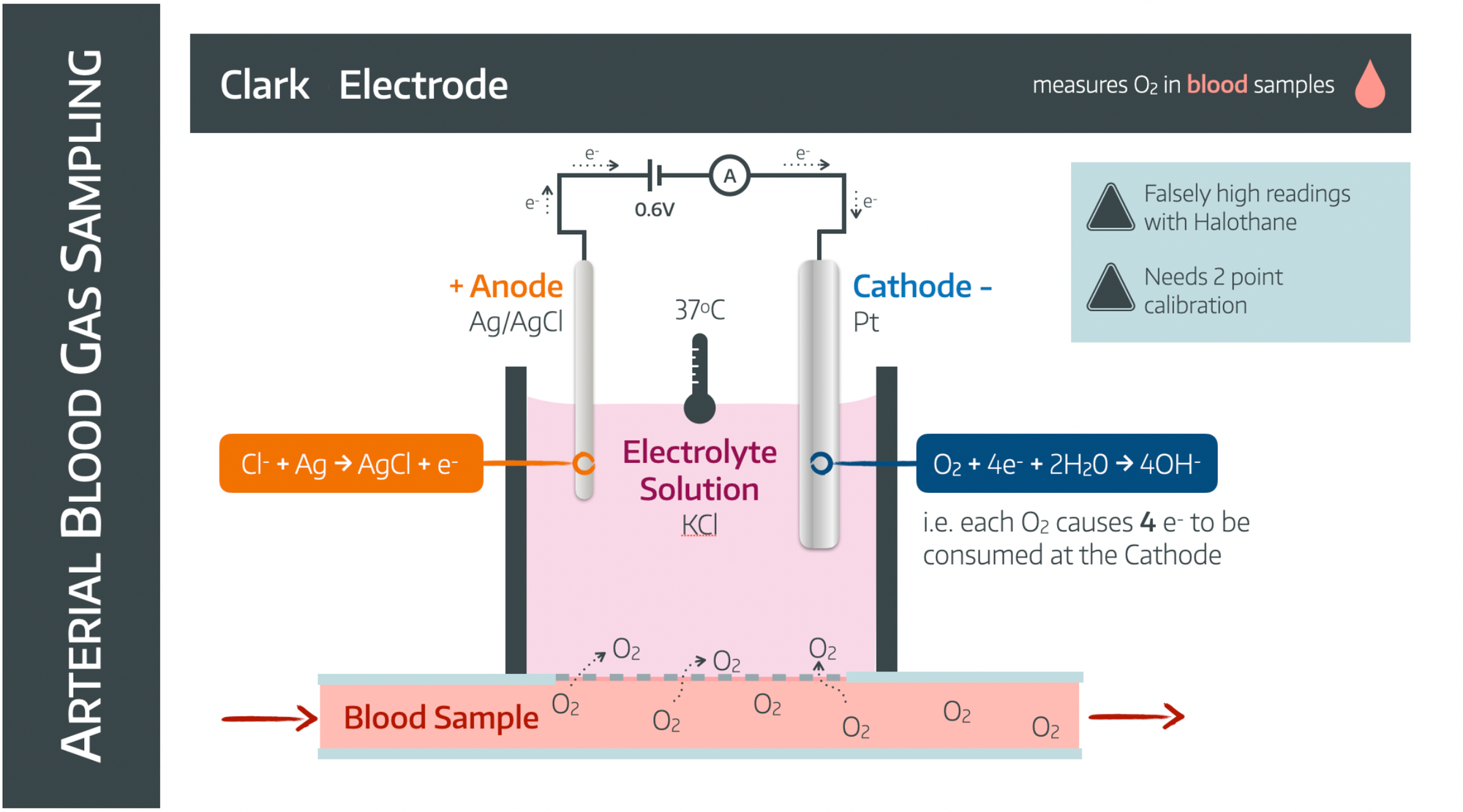
Hopefully this image makes a tad more sense now.
There's a video in the members section below as well.
A spot of history
Meet the father of biosensors, Leland Clark, the absolute legend you have to thank for the magic you've just witnessed.

In the 1950s he developed the first ever bubble oxygenator to be used in cardiac surgery.
Frustratingly, when he came to publish his results, his work was refused by the editors as it wasn't possible to measure the oxygen tension in the blood that the device was pumping out.
So Clark made a thing to do just that, in 1954.
Not only did his device quickly become the foundation of pretty much every ABG machine from the 1970s onwards, it also paved the way for other biosensors including the glucose sensors used by millions around the world today.
I'm interested in how this led to a glucose sensor
Oh magnificent I'm so pleased you asked.
So you start with a Clark electrode,
- You then stick glucose oxidase onto the O2-permeable membrane
- Then feed it a blood sample exactly as you would normally
- The glucose oxidase then catalyses oxidation of glucose
- This consumes oxygen
- The clark electrode then detects a drop in pO2, which is proportional to the amount of sugar present
Now substitute in lactate oxidase and you have yourself a lactate electrode too.
Neat.
Why 0.6V?
This is a sweet-spot voltage.
- There is a sigmoid relationship between the voltage you put in, and the current you get out
- At very low voltages, there isn't enough energy to reduce all the available oxygen
- Above 0.6V, there is enough energy to rapidly reduce all the available oxygen
- Hence above 0.6V limiting factor switches from voltage to oxygen tension
- This is therefore the most efficient voltage to use, because any more is unnecessary
Above 1V you also start reducing water molecules, which totally throw the measurements off.
Here's his diagram
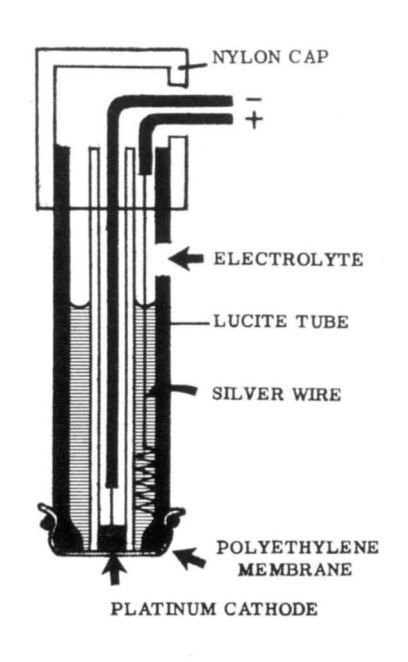
Here are some of his papers


Useful Tweets
History Fact Friday! In 1958, John W. Severinghaus developed a complete gas apparatus by combining his practical version of Stow's CO2 electrode with Clark's pO2 electrode. More: https://t.co/sWVRWU1Z5y pic.twitter.com/axoSxS9Gdq
— ASA® (@ASALifeline) July 27, 2018
References and Further Reading



Primary FRCA Toolkit
Members receive six months of free membership with the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.



