ABG and measuring gases
Take home messages
- You need to be able to draw the different electrodes and explain how they work
- They all generally work by measuring a voltage change due to ion concentration differences
How does an ABG machine measure pH?
It uses a pH electrode
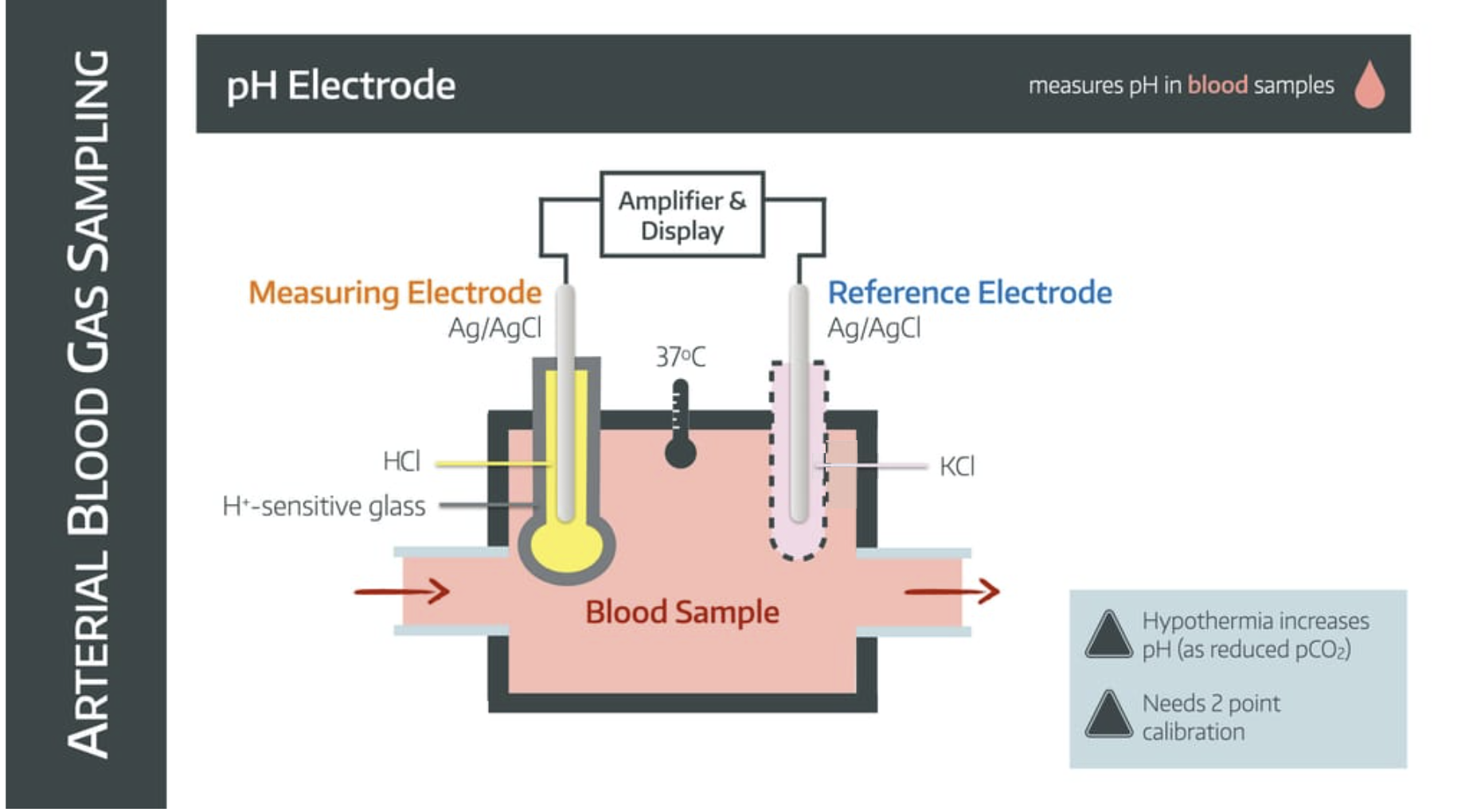
How it works
The blood sample enters a chamber with two electrodes suspended within it - the measuring electrode and the reference electrode.
The measuring electrode
- is made of silver/silver chloride
- is immersed in HCl buffer solution to maintain constant H+ concentration
- is separated from blood sample by ion-sensitive glass
The reference electrode
- is made of silver/silver chloride (sometimes mercury)
- is immersed in potassium chloride
- is connected via a semipermeable membrane to complete the circuit
The whole system is kept at 37°C.
The difference in H+ concentration between blood sample and buffer solution generates an electromotive potential across the glass.
This electrical signal is amplified and calibrated to give a pH value.
How does an ABG machine measure CO2?
It uses a Severinghaus electrode, which is exceedingly similar to the pH electrode.
In fact, it works in exactly the same way, it just has to figure out how to convert CO2 into something involving ions that can then cause a voltage change.
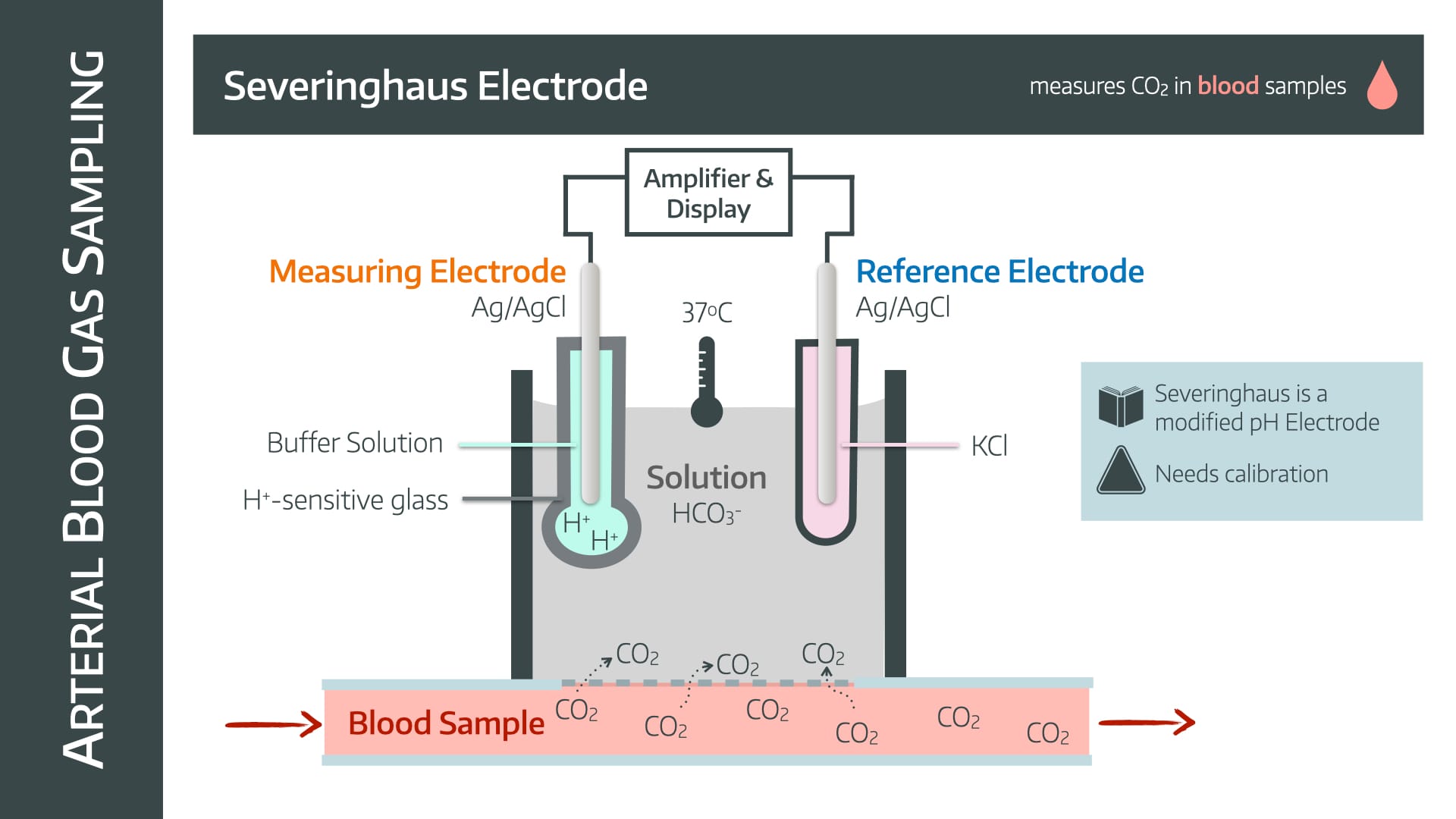
- CO2 diffuses from the blood sample across a CO2-permeable membrane into a bicarbonate solution
- This causes it to dissociate to form H+ ions
- The rest of the electrode then behaves in the same way as a pH electrode, but calibrates the change in pH to the PaCO2
- Because of the extra time needed to allow the CO2 to diffuse and equalise in solution, the Severinghaus electrode is relatively slow
- Needs calibrating and regular maintenance
How do the values change with temperature?
- Gas solubility in water increases as temperature decreases
- Therefore hypothermia will cause a lower PaCO2, and therefore increased pH
If you're a pH enthusiast then consider perusing our ridiculous podcast episode cover of this article by Dr Yartsev over at Deranged Physiology.
How does an ABG machine measure oxygen tension in a blood sample?
It uses a Clark electrode
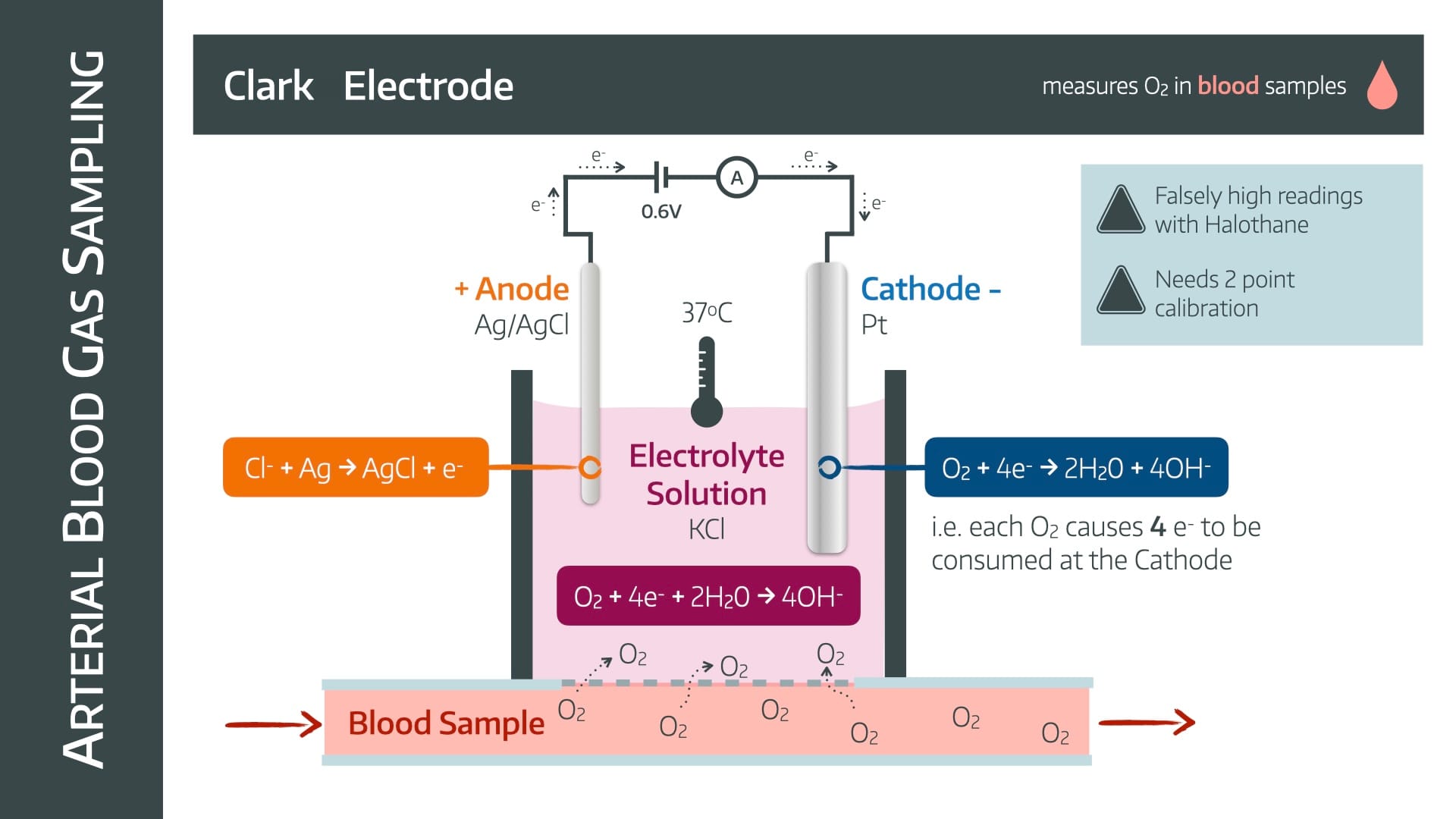
The oxygen diffuses across a semipermeable membrane, and reacts with the electrons at the cathode to produce hydroxide ions.
These hydroxide ions make the electrolyte solution slightly negatively charged.
This encourages the forward reaction at the anode as it attempts to retain electroneutrality by consuming negative chloride ions and churning out electrons.
Therefore as electrons are consumed at the cathode, they will be replaced by electrons flowing from anode to cathode, through an ammeter
The current will be proportional to the consumption of electrons, and therefore the partial pressure of oxygen.
How does an anaesthetic machine measure oxygen tension in a gas sample?
Measuring gas tensions in a gas sample requires a different approach to measuring the tensions of dissolved gases in a fluid.
Classically anaesthetic machines use either a paramagnetic analyser or a fuel cell, or both
Paramagnetic analyser
Paramagnetic substances are attracted to a magnetic field, compared to diamagnetic substances that are repelled.
Older paramagnetic analysers used a 'suspended dumbell' mechanism to measure oxygen tension, while newer analysers use a highly sensitive pressure transducer as described below
- The analyser has two chambers separated by a sensitive pressure transducer
- Room air is entrained into one chamber and the fresh gas flow to be analysed is directed into the other
- A magnetic field is generated across the testing chamber, which agitates the oxygen molecules causing an increase in pressure in that chamber
- The higher the concentration of oxygen, the greater the pressure increase as compared to the room air sample
- This is detected by the pressure transducer
This method has four advantages and three disadvantages
Advantages
- Fast
- Accurate
- Sensitive
- Works continuously
Disadvantages
- Needs a power supply
- Can suffer from interference such as from water vapour, so gas needs to be passed through a drying agent such as silica gel
- Needs calibration
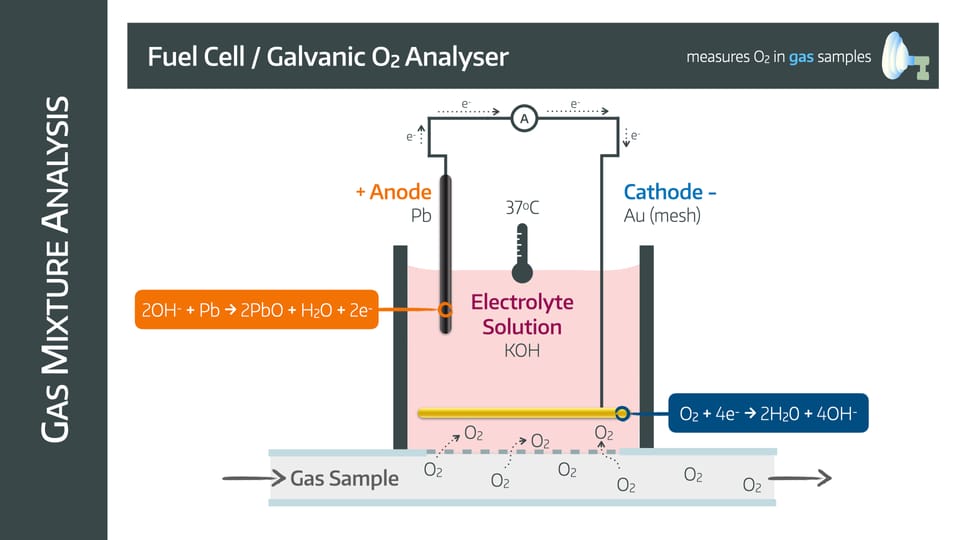
Fuel Cell
Also known as a galvanic oxygen analyser
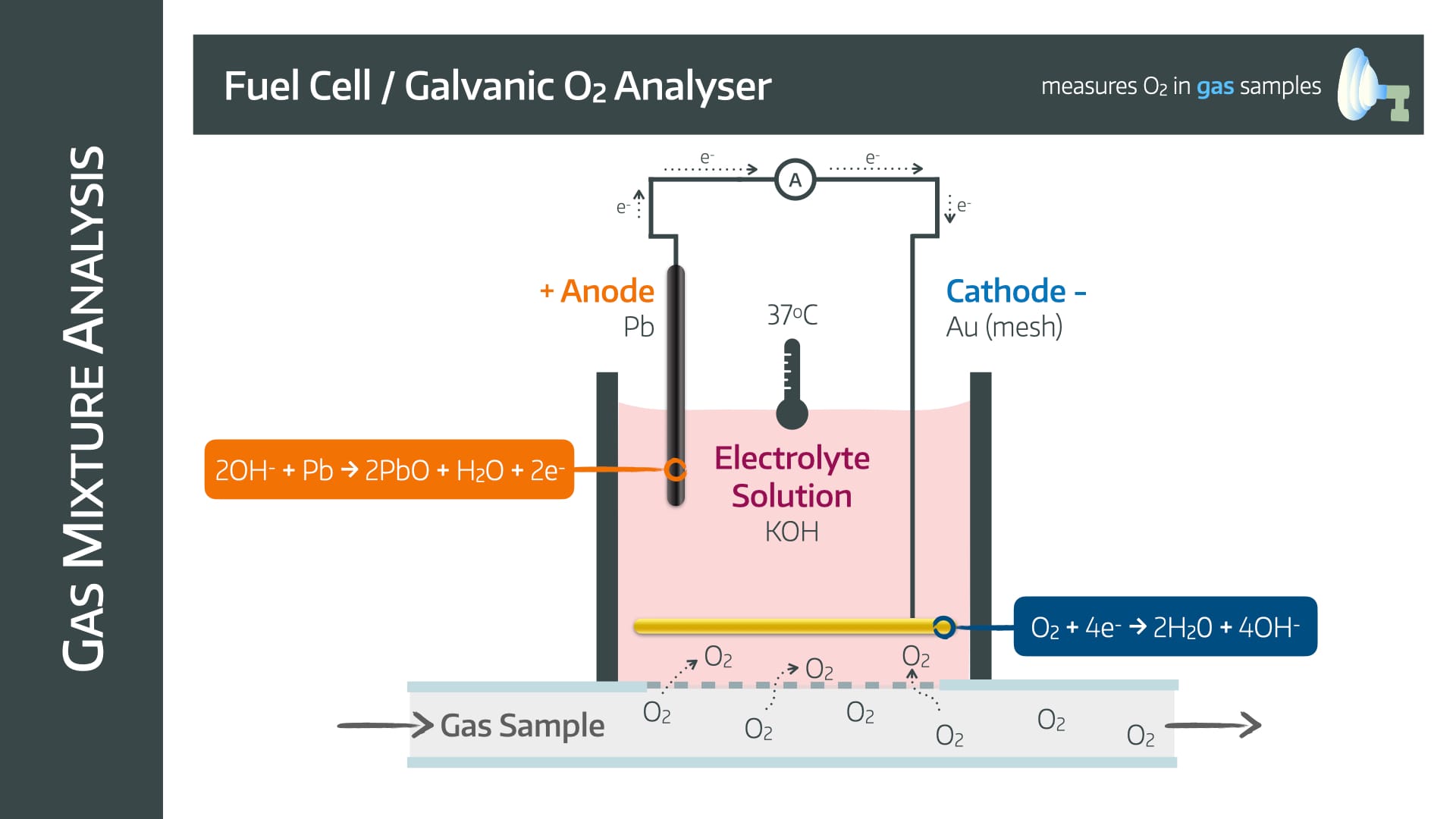
It has a lead anode and a gold mesh cathode in an electrolyte solution with potassium hydroxide surrounded by an oxygen permeable membrane
The mechanism relies on the following reactions

Advantages
- Cheap
- Compact
- No power supply required
- Unaffected by water vapour
Disadvantages
- Lead anode is consumed and needs replacing
- Needs regular servicing
- Nitrous Oxide can interfere with accuracy and damage electrodes
- Slow (takes upwards of 30 seconds)
Have you heard of the Haldane apparatus?
You just need to be able to briefly describe it
- By measuring the volume of a sample of gas before and after the removal of an anaesthetic volatile agent, it can work out the concentration of that agent
- Uses potassium pyrogallate absorbant to absorb oxygen
- Potassium hydroxide used to absorb CO2
- Not really used much any more
How can we measure concentrations of gases and anaesthetic agents in a gas sample?
We can divide this up into those used commonly in practice, those that are rarely used, and those only really used in research:
Commonly used in practice:
- Infrared absorption spectrometry - CO2, N20, Anaesthetic agents
- Paramagnetic analysers - Oxygen
- Fuel cell - Oxygen
Rarely Used:
- Piezo electric effect using quartz crystals
Stimulating quartz crystals with an alternating current makes them oscillate at their resonant frequency. The crystals are coated in oil to allow lipid-soluble volatile agents to dissolve onto the surface of the crystal. This changes the resonant frequency of the crystal, and the degree to which the resonance is changed reflects the concentration of the volatile agent.
This method cannot distinguish between different anaesthetic agents, nor can it measure oxygen, nitrous or CO2.
- UV absorption - Halothane
- Raman scattering
As a photon hits a gas molecule, some of the energy is absorbed and re-emitted at a different wavelength - the Raman effect. The absorption of energy at a different wavelength depends on the bonds between the atoms and therefore each gas will have a characteristic absorption profile.
Argon laser light is used to irradiate molecules and a set of filters specific to each gas is used to separate out the relevant wavelengths, allowing detection of each individual gas
- Mass spectrometry
Can identify many different compounds simultaneously, including water.
Only used in research:
- Gas chromatography
A mixture of gas is separated into components by passing it through a column with a stationary phase and a mobile phase.As the mixture passes through the column, the individual gases dissolve into the mobile and stationary phases to different extents, meaning they arrive at the other end of the column at characteristic time points.
Note that this method requires prior knowledge of the gases you are expecting, and pre-verified times at which they are expected to arrive at the end of the column
It is very accurate and sensitive but expensive and cannot do continuous analysis
- Photoacoustic spectroscopy
- Thermal conductivity detection using a catherometer
- Ultrasound velocity through gas mixture analysis
- Refractometers
What's a derived value?
Usually a blood gas machine takes a sample of around 200 microlitres and passes it through four different electrodes:
- A pH electrode
- A Clark electrode
- A Severinghaus electrode
- A reference electrode
All at a temperature of 37°C.
Analysers can also measure ion concentrations, lactate and haemoglobin.
Some parameters can then be deduced, or derived from the measured data, including:
- Bicarbonate
- Base excess
- Oxygen saturation
Not useful but funny
We asked ChatGPT to draw a pH electrode, and the resulting image is exactly what the pH electrode looks like when you're trying to learn it for the first time as a CT1 #FRCA pic.twitter.com/iQjPx4xPLP
— Anaestheasier (@Anaestheasier21) November 8, 2023
Syllabus
- PC_BK_78 Measurement of gas and vapour concentrations: e.g. infra-red, paramagnetic, fuel cell, oxygen electrode, mass spectrometry
- PC_BK_79 Measurement of pH, PCO2, PO2
- PC_BK_80 Derived blood gas variables, e.g. HCO3a, HCO3s, BE. Siggaard-Andersen nomogram
Useful Tweets and resources
References and Further Reading


Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.