Medical Gases

Take home messages
- Medical gas supplies are dangerous, so they have a whole host of safety features, which of course you need to be able to recite at a moment's notice
- The physicochemical properties of nitrous oxide and CO2 mean they're stored differently to the other gases
Podcast Episode
Anaesthetists like gas
And we're even bigger fans of vapours.
Pretty much every doctor uses oxygen at some point, and many will dabble with a pinch of entonox now and again, but the regular use of multiple gases and vapours remains the remit of the anaesthetist, and for good reason - because they're dangerous.
Gases we commonly use or encounter:
- Oxygen
- Carbon Dioxide (CO2)
- Nitrous Oxide (N2O)
- Medical Air
- Entonox (50:50 mix of Nitrous Oxide and Oxygen)
- Heliox (Mixture of helium and oxygen - between 60:40 to 80:20)
A whiff of history
Oxygen is wrong. By which I mean the name is wrong.
Technically speaking we should say dioxygen, because oxygen means the single atom 'O' with an atomic weight of 8.
The name also comes from the Greek “oxys,” - which means “sharp” as in how a lemon tastes - and “-genes,” which means “begetter.” This is because Lavoisier believed it was an essential ingredient in giving all acidic substances their 'spice' for want of a better word.
'Begetter' is quite good, however, as it does rather emphasise the fundamental importance of oxygen for everyday life.
When was it first discovered?
1771.
Not by Joseph Priestley, as many people believe.
To give him due credit, he did successfully produce oxyen in a laboratory when he heated red mercuric oxide, producing oxygen which caused a candle to burn with more vigour.
However this was three years after Carl Wilhelm Scheele, a German-Swedish chemist, heated mercuric oxide, silver carbonate and a bunch of other bits and bobs and produced something that he subsequently gave the wonderful name of 'fire air'.
Priestley was “first to press,” publishing his generation of oxygen in 1774, and has since often been credited with the original discovery.
It took until 1868 to stick it in a cylinder and use it for anaesthesia, and 1885 before its use in helping patients with pneumonia was properly appreciated.
I won't natter on - If you'd like to read more, find the article of reference here
Let's start with some definitions.
A cylinder of 'medical gas' is filled with a fluid - which can be a liquid, vapour or gas.
By definition, a vapour is a substance that has evaporated, so it's no longer a liquid, and the molecules are behaving like a gas. But if you were to apply enough pressure, you could squish it back into a liquid if you like.
There is a temperature above which you can no longer compress this substance back into its liquid form, no matter how much pressure you apply, and this temperature is the critical temperature.
By definition, above this temperature it becomes a gas.
(If you're interested, Nitrous Oxide has a critical temperature of 36.5°C, meaning it is inhaled as a vapour, then warmed to body temperature and exhaled as gas, with precisely zero clinical relevance.)
If you have liquid in a partially-filled cylinder, then some of this liquid will evaporate into its vapour phase to fill any remaining space.
As more of it evaporates, the pressure this vapour exerts will increase, until the point where there is so much pressure that no more molecules can evaporate.
At this point we say the remaining space is saturated with vapour, and the pressure it exerts is called the saturated vapour pressure.
If you warm the liquid up, then the molecules have more energy and can evaporate despite higher pressures, so this saturated vapour pressure increases.
It's also why it is a bad idea to heat up a cylinder full of medical gas in its liquid form. Or at all really.
The critical pressure is the saturated vapour pressure at the critical temperature, beyond which the molecules will remain in the gaseous phase.
What kind of pressures are we talking?
Big ones.
Like - 137 atmospheres big.
Some perspective:
- Car tyre pressure - 2 atm
- DM-41 hand grenade - 2.6 atm
- Road bicycle tyre - 6 atm
- The oxygen cylinder you're holding - 137 atm
- Water pressure at depth of Titanic - 400 atm
- Bottom of Mariana Trench - 1100 atm
What is the definition of pressure and gauge pressure?
Pressure is a measure of the force per unit area
- 1 pascal = 1 newton per metre squared
- 1 atmosphere = 101.3 kPa = 760 mmHg = 1033 cmH20 = 1.013 bar
- Gauge pressure is the difference between absolute or total pressure and atmospheric pressure
- Absolute pressure = Gauge pressure + atmospheric pressure
- An ‘empty’ gas cylinder has a gauge pressure of zero, but actually contains air at atmospheric pressure, so the absolute pressure in the cylinder is 1.013 bar
Is it safe?
I'll admit I get a little uneasy around gas cylinders. There's something about all that pressure inside a relatively thin-walled container that's just a smidge too grenade-y for my liking.
Fortunately, however, cylinders and gas supplies have a whole array of safety features to reduce their explosive tendencies and make them very efficient ways of storing vast quantities of useful gas.
This next question comes up a lot, particularly in the FRCA Primary OSCE exam, so it's good to have a slick answer up your sleeve.
What safety features are found on medical gas cylinders?
As always, you're going to categorise first, then give details later. There are four main categories for this answer:
- They're colour coded
- They have a pin-indexing system
- They have a filling ratio
- They have specific manufacturing and maintenance requirements
- Many have safety pressure release valves as well
Then once you've given them these categories, you can delve into a little more detail if required.
Colour coding system
- Oxygen cylinders are white
- Nitrous oxide cylinders are 'French' blue
- Medical air cylinders have a black and white collar
- Entonox has a blue and white collar
- Helium is brown
- Heliox is brown and white
- CO2 is grey
Note that the collar is the most important part. Some nitrous cylinders for example have different body colours, but the collar is always French blue.
The pin indexing system
This lock-and-key mechanism essentially prevents you attaching the wrong cylinder to the yoke on an anaesthetic machine, and inadvertently pumping nitrous oxide instead of oxygen.
- Pins on the yoke must align with holes on the cylinder valve block
- There are six pin positions
- Oxygen has positions 2 and 5
- Nitrous oxide uses positions 3 and 5
- Air uses positions 1 and 5
This isn't the most enormously high yield set of numbers to learn, but if you're super keen, you can remember it as all of them using position 5, then 'Air' having 1 syllable, oxygen having 2 atoms in a molecule and nitrous having 3.
Filling ratio
As we mentioned earlier, it's probably best not to fill a cylinder full of liquified gas all the way to the brim, because liquids aren't compressible, and so any increases in temperature are likely to result in enormous pressures on the walls of the cylinder.
To counter this, cylinders have a filling ratio, which is defined as
- The maximum safe weight of liquefied gas in the cylinder, compared to the weight of water that would completely fill the cylinder at 15ºC
- This is 0.75 in cooler regions
- 0.67 in warmer climates
Manufacture and maintenance requirements
- Cylinders are generally made of molybdenum steel, which is really strong
- Some others are made of composites, and sometimes wrapped in kevlar
They also have to undergo BOC-approved testing:
- Inspection inside and outside every year
- Hydraulic testing to 50% above working pressure
- Every 5 years for composite cylinders
- Every 10 years for steel cylinders
- A plastic ring around the neck indicates the next test date
- The BCGA (British Compressed Gases Association) recommends pressure regulators are refurbished or replaced every five years
Okay so what about the piped gases?
Most hospitals have a piped wall supply of oxygen, medical air and often nitrous oxide as well (usually just to the anaesthetic machines in theatre).
The piped oxygen supply is most commonly provided by the vacuum insulated evaporator or VIE.

How does the VIE work?
We made a video here
The vacuum insulated evaporator is essentially an enormous thermos flask that stores liquid oxygen with its vapour phase above the liquid, which is delivered regularly by a tanker from the nice people at BOC.
What is the boiling point and the critical temperature for oxygen?
- Boiling point is -185°C
- Critical temperature is -118°C
- At this temperature, 5000kPa is required to keep it in liquid form - this is the critical pressure
- Above this temperature, oxygen cannot be compressed back into its liquid form, no matter how much pressure is applied
It is for this reason that the VIE maintains a pressure of around 10 atmospheres or 1000kPa and a temperature of -150°C
- As the name suggests, it has a vacuum insulating layer around it to maintain such a low temperature
- It then uses the latent heat of vaporisation maintain a temperature of -150°C
- As oxygen vapour is drawn off to be used by the hospital, more liquid oxygen is able to evaporate to replace it, which is an endothermic process
- It maintains an internal pressure of up to 10 bar, above the pressure required to keep oxygen in its liquid phase, and high enough to facilitate more efficient storage of oxygen
- Because it contains liquid oxygen with the vapour phase above it - much like nitrous oxide cylinders - a pressure gauge won’t reliably inform you of the amount of oxygen left, as it will simply read the saturated vapour pressure right up until the point where all the liquid oxygen has evaporated
- Therefore the whole device sits on a set of scales which is used to work out how much liquid oxygen remains in the system
- Newer VIEs also have differential pressure transducers that compare the pressure at the bottom and the top of the container
Where does the oxygen come from?
Usually a large truck from the nice people at BOC.
How is medical oxygen manufactured?
You can produce oxygen in a variety of ways but the most common method, which you need to be able to recite for the exam is Fractional Distillation of Air.
Essentially air is cooled way down below the boiling points of its constituents so it liquefies, and then it is slowly allowed to warm up again.
As each constituent reaches and exceeds its boiling point, it will evaporate off.
Helium will evaporate off above -269°C (if you even managed to liquefy it in the first place)
Nitrogen will then evaporate above -195.8°C
This will then leave liquid oxygen behind.
Other methods:
- Heating barium oxide to 800 degrees celsius, but this is inefficient
- Oxygen concentrators
Oxygen concentrators use aluminium silicate or zeolite to adsorb nitrogen from air, producing up to 93% oxygen. These are cheap and reliable and avoid the need for an oxygen supply.
What pressures are delivered via the wall supply?
- Gases are supplied at 400 kPa (4 bar)
- Medical air is supplied at 700 kPa (7 bar) but only when used for driving specific surgical equipment
What safety features do the piped gas hoses have?
Yes. This is a question that comes up.
First and foremost, they are colour coded:
- Oxygen is white
- Nitrous is blue
- Air is black
- Suction is yellow
- Scavenging is colourless
They are also reinforced and non-compressible
They are also unique to each gas:
- Schrader valve at wall end
- Non Interchangeable Screw Thread at anaesthetic machine end with probe size unique to each gas
Make sure you say the bold words in the exam.
What prevents dangerously low levels of oxygen from being delivered to the patient?
In the olden days, there was a CO2 cylinder on the back of the anaesthetic machine, which could be used to raise the FiCO2 delivered to the patient in order to get them breathing sooner.
Because Murphy's law exists, however, this means that several patients ended up receiving disastrously high levels of CO2 or low levels of oxygen for whatever reason or mistake, so it was agreed we should probably stop.
Nowadays the anaesthetic machine has several ways in which to prevent a dangerously hypoxic mixture being delivered to the patient.
- Oxygen failure alarm - If the pipeline supply drops below 200 kPa
- Back up oxygen supply - From the wall oxygen supply
- Secondary back up - Cylinder on anaesthetic machine
- Dual monitoring of FiO2 - both ventilator and monitor screens, each with an alarm if FiO2 drops below 21%
- Hypoxic guard - the nitrous and oxygen rotameter on older machines are mechanically linked to ensure nitrous is not delivered without sufficient oxygen
Why Nitrous and CO2 are a little different
Nitrous oxide and CO2 have rather high critical temperatures of 36.5°C and 31°C respectively, when compared to gases like nitrogen and oxygen.
This means storing them under substantial pressure at room temperature (15-20°C depending on who you're listening to) means they tend to liquefy in the cylinder.
There will always be some evaporation of the liquid into the vapour phase, which is what leaves the cylinder when you open the tap to use the substance, but once the tap is closed again, the lost vapour is quickly replaced by newly-evaporated molecules.
- As a result, the pressure inside the cylinder will remain at the saturated vapour pressure for the current ambient temperature
- At room temperature this is approximately 4400kPa for nitrous oxide and 5700 kPa for CO2
- This will continue until enough vapour has been removed to allow all of the remaining liquid to evaporate, leaving only vapour in the cylinder, at which at which point the pressure on the gauge will drop rapidly as the last remaining vapour is used
- This means that the quantity of nitrous oxide or CO2 in the cylinder cannot reliably be measured using a pressure gauge, as it will simply read a constant value (the SVP), right up until the very end, and then drop very rapidly when the last vapour is used
- Therefore nitrous oxide and CO2 cylinders are weighed and compared to their tare weight to determine how much liquid remains
Tare weight is the weight of the empty cylinder
How many oxygen cylinders should I take?
Transfer medicine is scary because lots of things can go wrong with a critically ill patient, even more so when you're on your own going 70mph in a box.
You don't want to run out of anything, least of all oxygen.
Thankfully most ambulances have enormous oxygen supplies so it shouldn't be an issue, however there are always the scenarios, particularly in SIMworld, when the ambulance supply fails, and you need to know that you've got enough back ups to deal with the situation.
How much oxygen should you take with you on transfer?
You should calculate your oxygen requirement, then double it
- Oxygen requirement = usage (litres/minute) x length of journey (minutes)
- Usage = Minute ventilation (litres/minute) x FiO2 (%) + Ventilator use (litres/minute)
Some ventilators can use air rather than oxygen, which removes the last term - check which model you're using
A useful table
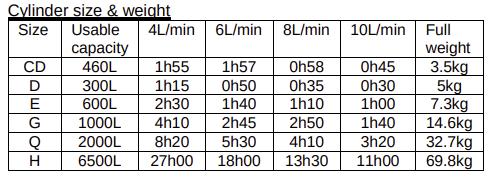
An OSCE question
What is the volume of oxygen in each size of oxygen cylinder?
- C - 170 litres
- D - 340 litres
- CD - 460 litres
- E - 680 litres
- F - 1360 litres
- G - 3400 litres
Syllabus
- PC_BK_23 Manufacture and storage of gases and vapours, safety
- PC_BK_24 Cylinders and pipelines, Bourdon gauge
Useful Tweets and Resources
Love these bite size bits of useful learning from @ChaseSurgical. Medical gas pin indices feature in the FRCA exams too. 😷😀 pic.twitter.com/Vi2VFFbNST
— Richard Morse (@RegMorse) December 11, 2021
Physics Gif Friday: when the air pressure inside the tank is lower than the atmospheric pressure outside it implodes pic.twitter.com/IuBYwA86vX
— Institute of Physics (@PhysicsNews) January 8, 2016
References and Further Reading



Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.


