Renal Replacement Therapy
As we've said before here at Anaestheasier, one of the things people tend to misunderstand about ICU is that we don't really fix all that much, we just buy the patient time.
Now, of course we treat them for nasty things, with fluids, antibiotics, steroids etc but that's all stuff you can quite easily do on a normal ward.
The things that are unique to ICU - vasopressors, ventilators, ecmo, dialysis and medically induced coma - none of these really fix anything, they just replace or stimulate the relevant organ system for a while, in the hope that the patient has the physiological strength to overcome whatever pathology has brought them to the not-quite-pearly gates of ICU in the first place.
And renal replacement therapy is no different.
Take home messages
- Up to 60% of ICU patients will get an AKI
- 2/3rds of these will require renal replacement therapy
- The mortality of AKI on ICU is between 10 and 60%
Keep it simple
The kidneys aren't cutting the mustard, and you've decided to help out with some renal replacement therapy, meaning you're going to try and replicate their usual functions with some sort of machine, and buy time to allow them to get better (or to wait for a transplant).
So what do the kidneys do?
- Good stuff in, bad stuff out
- Fluid balance management
- Acid-base management
- Hormone synthesis
- Red blood cell production
- Vitamin D production
We can only really replace the first three, and that's where our fancy looking urine-Dalek comes in.

A spot of history
Meet Scottish chemist Thomas Graham, whose name you'll recognise from the gas laws of diffusion.
Well this apparently wasn't keeping him busy enough so he also first conceptualised the idea of dialysis using parchment way back in 1861.
American pharmacologist John Jacob Abel then thought it might be possible to help patients with kidney disease by circulating their blood through some form of filter.
It wasn't until 1924, however, when Georg Haas took a crack at dialysing a human patient in acute renal failure. It didn't work, mind you, but hey - it started something great.
Oh, and heparin wasn't a thing prior to 1922, so they had to crush the heads of leeches to get hold of the rather toxic hirudin instead, which had mixed effects to say the least.
It was 1945 before the first patient with acute renal failure was successfully kept alive with haemodialysis by Willem Kolff.
Here's a simply wonderful video from 1982 on the history of dialysis
So when should I do it?
As anaesthetists, we're usually involved in an emergency ICU context, so we're mostly talking about emergency indications for putting a splendidly large central line in and plumbing the poor patient into some complex machinery.
This is not a risk-free procedure - it is invasive, expensive and carries substantial potential for badness if not done correctly - so specific indications for the treatment have to be met if one is to justify said risks.
What are the indications for emergency renal replacement therapy on ICU?
- Hyperkalaemia refractory to medical management (at least three proper rounds of management)
- Fluid overload refractory to medical management
- Acidosis refractory to medical management
- Symptomatic uraemia (encephalopathy, pericarditis or haemorrhage)
- Specific toxin removal (drug overdose)
- Patient already on dialysis requiring emergency filtration*
*This is classically referred as something along the lines of
"Mr Jones usually has Monday, Wednesday, Friday dialysis, and he was too unwell to attend his Friday session due to his pneumonia, he's then waited in ED for a while and now it's Saturday night and he's not well and please can you help..."
Totally reasonable - admit to ICU and stick him on the filter until Monday morning when you can talk to renal and see if they're able to get him back onto his usual dialysis regime.
Bosh.
In general, the evidence seems to suggest that patients that require RRT do better if they're filtered early, so if you think a patient is headed towards the filter, probably best not to hang around.
Where should I put the vascath?
According to KDIGO:
- RIJ is best choice
- Then femoral
- Then LIJV
- Then subclavian
Subclavian carries higher risk of stenosis formation.
The optimal flow rate is achieved when the tip of the vascath is in the right atrium (for IJV) or inferior vena cava (for femoral).
So what type should I use?
Some definitions to start
- Haemofiltration - I push the blood through a semi-permeable membrane that lets plasma and small stuff fall out but keeps the big stuff
- Ultrafiltrate - the mixture of plasma and small molecules that is successfully shoved through said membrane and discarded (don't worry, the good stuff and fluid gets replaced later)
- Dialysis - I run a dialysate fluid next to the blood, again with a semi-permeable membrane between the two, allowing the two fluids to equilibrate their concentration gradients of a variety of substrates - bad stuff out, good stuff back in
- Haemodialofiltration - mindblowingly, a combination of the two, where an ultrafiltrate is produced and discarded with the used-up dialysate fluid
And a smidge of physics
- Diffusion - spontaneous movement from high to low concentrations
- Convection - solute is dragged through a membrane by the ultrafiltrate
- If the solvent passes through a membrane, but the solute doesn't, it's osmosis
- If the solute passes through, then it's dialysis
You essentially have three options to clean someone's blood sans kidney:
- Intermittent haemodialysis
- Continuous haemofiltration or haemodialofiltration
- Peritoneal dialysis
We generally use continuous renal replacement therapy, rather than dialysis, on ICU because critically ill patients usually aren't able to deal with the haemodynamic insult of rapid dialysis.
Peritoneal dialysis is not commonly used in ICU as it's not very effective and messes with ventilation by increasing abdominal compartment pressure.
Occasionally you might find the stable chronic dialysis patient having a quick spin before returning to their usual satellite unit after they leave intensive care.
It doesn't seem to matter - in terms of mortality - what type of RRT you use, as long as the patient is able to tolerate the haemodynamic effects and they don't suffer any major complications of the therapy itself.
Continuous VV Haemofiltration
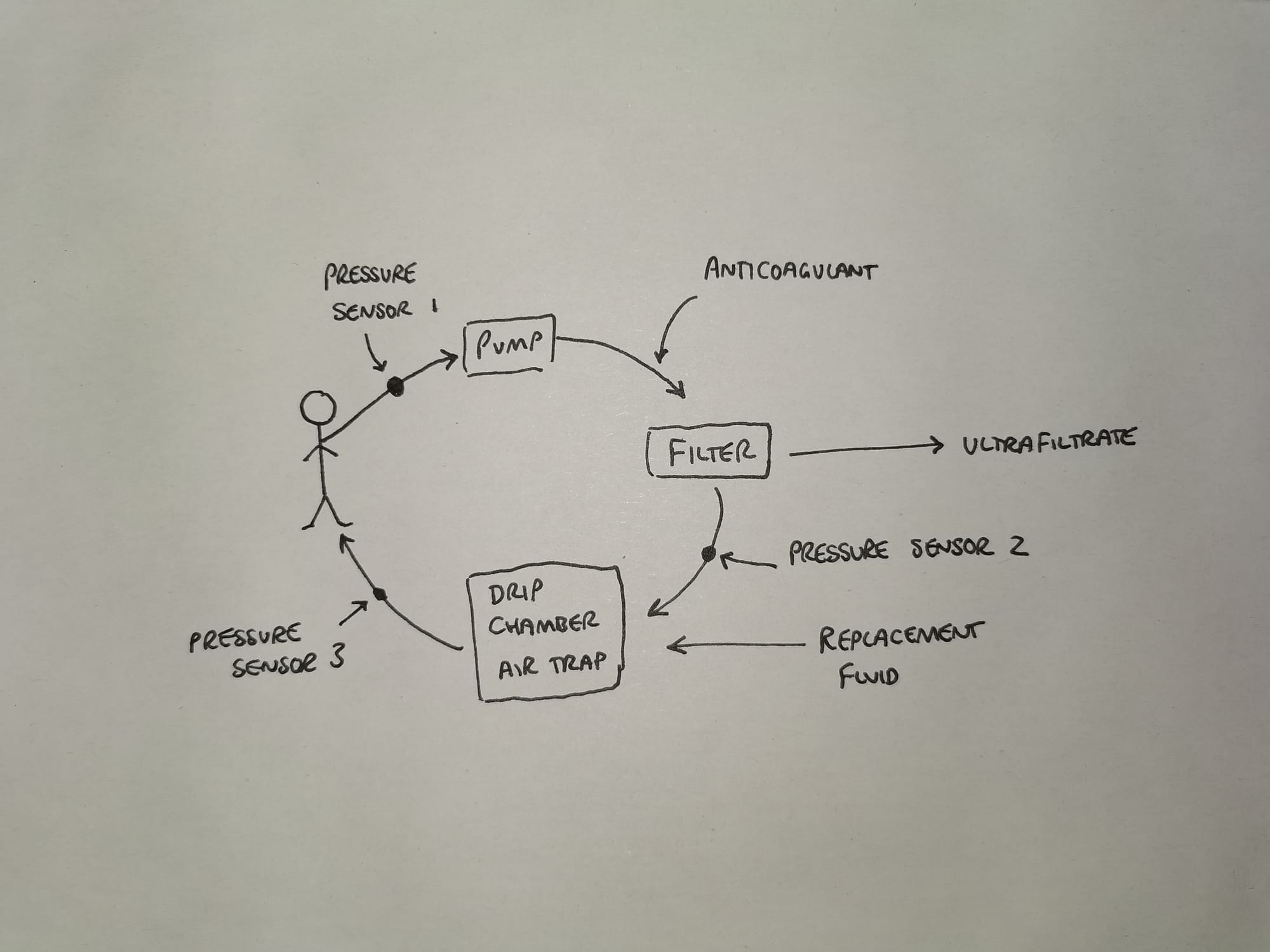
- Blood is pumped through the extracorporeal circuit and haemofilter
- This produces ultrafiltrate which is then replaced with replacement fluid depending on desired fluid balance
- Pressure sensor 1 detects whether the venous access is low (such as a blocked vascath, or very dehydrated patient)
- Pressure sensor 2 detects whether the filter is blocked or if clot is forming in the drip chamber
- Pressure sensor 3 detects whether the return lumen is blocked
Continuous VV Haemodialofiltration
CVVHDF is very similar, the only addition is that of a countercurrent dialysate flow through the haemofilter.
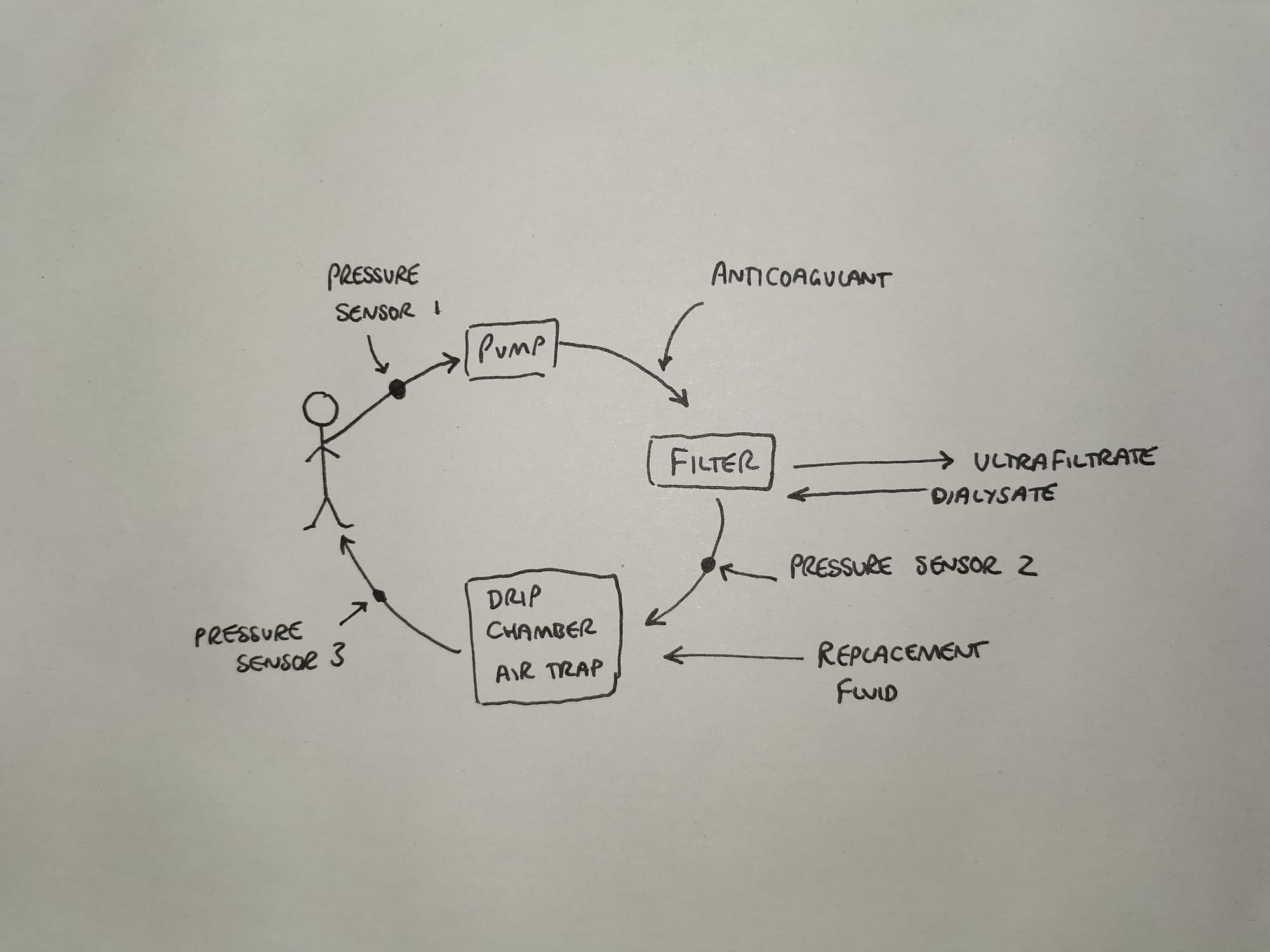
Intermittent Haemodialysis
Pretty much exactly the same set up as above, except the blood flow is higher and the dialysate flow is also much higher.
This produces large volumes of filtrate very quickly, and the rapid solute removal generally means less fluid is lost, and no replacement required.
The key point is we don't tend to do this on ICU because the patients aren't well enough to tolerate the cardiovascular insult.
Some FRCA Questions
What are the advantages and disadvantages of intermittent haemodialysis?
Advantages
- Generally readily available
- Simple to set up and use
- Rapid fluid removal
- Rapid solute correction
Disadvantages
- Most critically ill patients won't tolerate the haemodynamic effects of rapid fluid removal
- Renal ischaemia due to hypotension
What are the advantages and disadvantages of CRRT?
Advantages
- Cardiovascularly stable
- Effective steady fluid removal and solute correction
- Facilitates TPN by avoiding fluid overload
Disadvantages
- More expensive
- Slower than dialysis
What are the advantages and disadvantages of peritoneal dialysis?
Advantages
- Cheap
- Easy
- Doesn't cause haemodynamic compromise
Disadvantages
- Slow and inefficient at removing fluid and solute in large volumes
- Generally contraindicated on ICU as increases intra-abdominal pressure and worsens ventilation
The in depth bits
This machine is going to go boop at you on a night shift, and you might want to know why it's going boop, and more importantly - how to stop it going boop.
Normally the exceedingly experienced and highly efficient ICU nurses will sort it all out before you even realise there's an issue, but it's helpful to know and be able to at least try and troubleshoot a little yourself.
So here goes.
There currently isn't strong evidence to suggest any benefit to increasing this dose, so 20-30 ml/kg/hour is generally sensible.
Pressure issues
A common complaint from the machine is about 'access pressure'.
- Any kind of Flow = Pressure difference/resistance
- The pressure difference here is the access pressure - the return pressure
The access pressure is determined largely by
- Central venous pressure
- The location of the vascath or access point
- How fast the blood is being shoved along by the blood pump
So often if a patient has low access pressure, it's because they're hypovolaemic, or the vascath is in a suboptimal location.
Resistance issues
Flow through any tube is described by the Poiseuille equation, which can be rearranged to give:
- Resistance = (8ηl) / πr4
- r = radius
- η = viscosity
- l = length of the tube (dialysis circuits are usually around 3-4m)
The only two you can really adjust here are the radius (bigger tube, and ensuring there aren't any clots obstructing the lumen) and the viscosity (haematocrit, temperature, lipid content
A steadily increasing circuit resistance is suggestive of clot build up and suggests you may need to change the circuit.
You absolutely do not need to know this.
Important physical factors at play in dialysis
- Membrane thickness
- Membrane porosity
- Membrane surface area
- Counter-current mechanism
- Concentration gradients
- Diffusivity coefficients (determined by gas constant, viscosity and solute size)
- Solute particle size and charge
These are all words you can blurt out in an SOE/OSCE to try and scrape some more marks.
Don't waste any time going into this level of detail, you should be doing some physiology or reminding yourself why the vaporiser still works on top of a mountain.
What on earth is filtration fraction?
- This is the how much plasma is removed from the blood during filtration
If you have a higher blood hydrostatic pressure, a lower blood oncotic pressure , and a more negative ultrafiltrate pressure, then the filtration fraction will increase.
- The ideal filtration fraction is around 20-25% for a patient with a haematocrit of around 30%
Higher than this and you get tend to get clotting at the filter.
What types of membranes are there?
- Cellulose-based (dialysis)
- Synthetic (haemofiltration)
Cellulose membranes are rather more pro-inflammatory and so generally synthetic ones are used in critical care settings.
What are the components of a CRRT circuit?
- An extracorporeal blood circuit (4m of plastic tubing)
- A filtrate circuit
- A replacement fluid circuit (if using haemofiltration)
- Blood pump (usually some form of roller pump)
- Semipermeable membrane/filter
- Dialysis and/or replacement/substitution fluids
- Effluent collection system
- Anticoagulation
- Air bubble trap
- Pressure alarms
Generally speaking these circuits can flow at up to 700ml/min, giving ultrafiltrate production of around 20-35 ml/kg/hour.
You then replace the ultrafiltrate depending on what fluid balance you're trying to achieve.
Which solutes are removed by haemofiltration?
- Generally anything water soluble and not particularly protein bound up to a molecular mass of 20kDa
Traditionally we didn't actively replace the bicarbonate, because it has a ridiculously short shelf life, and it also makes calcium precipitate out of solution.
Instead we would give lactate, which is then metabolised to bicarbonate in the liver by the citric acid cycle (clearly this requires the liver to be working).
A normal liver can cope with around 100mmol of lactate per hour.
A critically ill liver might not, hence we now have bicarbonate-containing replacement fluids to prevent lactate overload.
Anticoagulation
Blood doesn't like plastic, or metal, or air.
You have three options to stop the blood forming large volumes of clot in your very expensive equipment:
- Do nothing - if the patient is already anticoagulated via their pathology or drugs
- Just anticoagulate the circuit (citrate)
- Anticoagulate the whole patient and circuit (heparin)
Citrate (the stuff in blue top blood bottles) is increasingly commonly used for its simplicity and avoiding having to anticoagulate the whole patient.
The citrate is added to the newly extracoporealised blood, and then reversed with calcium in the soon-to-be-intracorporealised (?) lumen.
You just have to keep an eye on the patient's calcium levels.
What are the risks?
- Bleeding (either from trauma to blood vessels or anticoagulation)
- Venous thromboembolism
- Circuit blockage
- Disseminated intravascular coagulopathy
- Metabolic disturbances as a result of giving too much or too little citrate
Will it affect other drugs?
Probably.
Each drug will have its own characteristic behaviour patterns, with some medications being highly dialysable and others not so much, depending on
- Protein Binding (less protein bound = more filtered out)
- Volume of Distribution
- Molecular Weight (big molecule = less filtered)
- Charge (to some extent)
So depending on these factors, as well as what method of RRT you're using and in what dose, you may have to adjust your dosing, timing, or monitor levels more often.
Long story short - check your local guidelines.
Useful resources
Another amazing infographic from Dr Nick Mark over at onepagericu.com
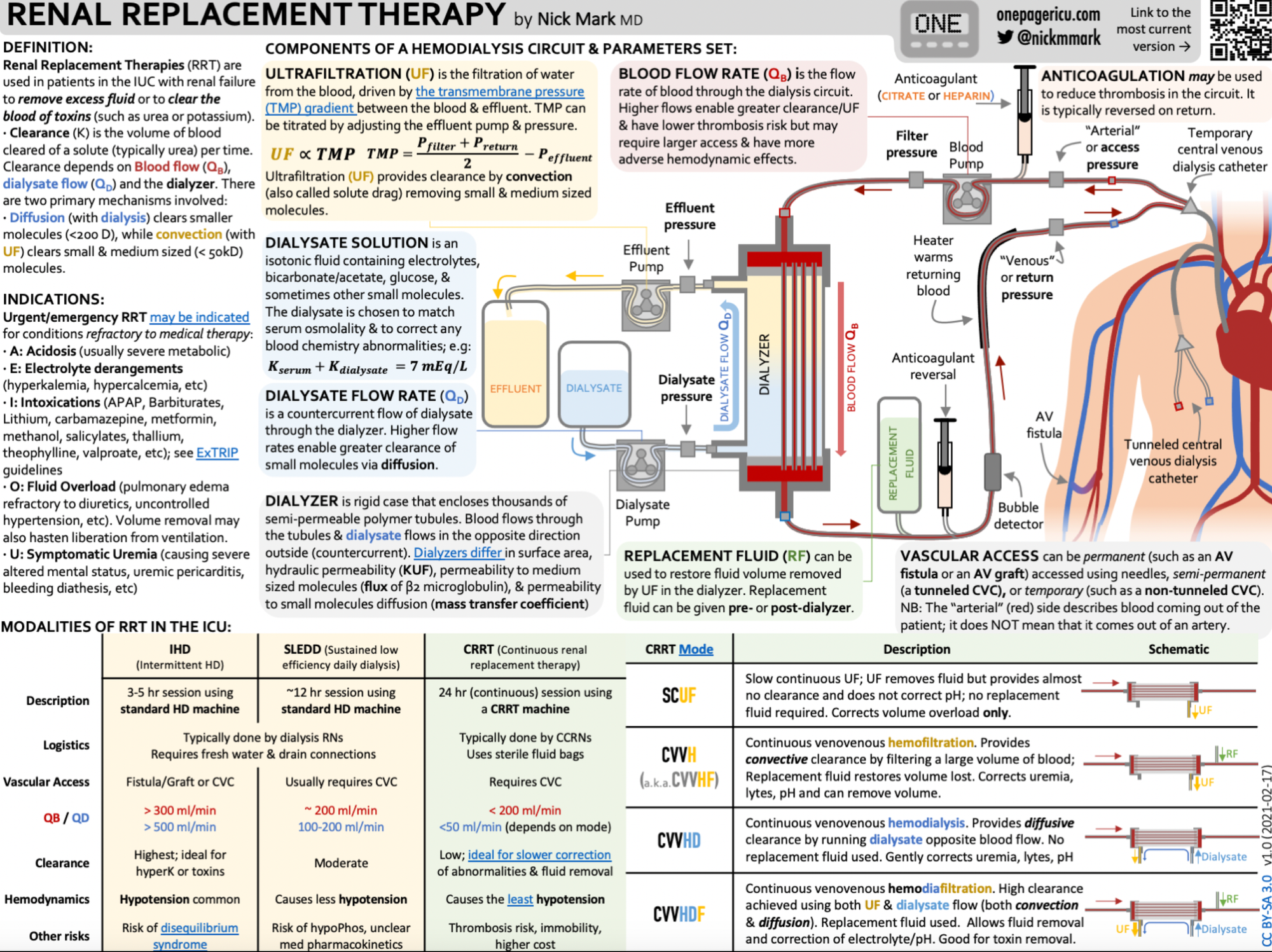
References and further reading


Members Area
This topic has come up twice in recent years in the Final CRQ paper, with the same themes being examined each time.
Here are the all highest yield questions for the exam.
