MAC and vaporisers

Take home messages
- Saturated vapour pressure is affected by temperature but not ambient pressure
- A vaporiser delivers a controlled partial pressure of volatile anaesthetic agent
- MAC is the end-tidal concentration of vapour required for 50% of patients not to flinch to a standard surgical incision
Podcast Episode
Here's our intro to how vaporisers work
@anaestheasier It all boils down to SVP
♬ original sound - Anaestheasier
What are vaporisers, and how do they work?
Let’s start with a liquid.
And we’re going to start with a volatile liquid, meaning it’s very keen on evaporating. In any given liquid, by random chance some of the molecules on the surface will have sufficient energy to break free from their surfance tension and intermolecular bonds, and escape into the air above.
These molecules are now in their vapour phase, wherein they’re behaving exactly like a gas, however at some point, some of these molecules are, by chance, going to lack sufficient energy to remain in the vapour phase, and so will duly condense back into the liquid below.
A quick recap on critical temperature
A substance can be in one of three broad phases:
- Solid
- Liquid
- Gaseous
A vapour exists when a substance is in its gaseous phase but below its critical temperature.
The critical temperature is the temperature above which a gas cannot be compressed back into a liquid, no matter how much pressure you apply.
Nitrous oxide has a critical temperature of 36.5°C.
This means that below this temperature, applying a whole load of pressure will squish it into a liquid, but above this temperature, it'll stubbornly remain as a gas no matter how much pressure you apply.
You might notice that this means you technically inhale nitrous as a vapour, but exhale it as a gas after warming it to body temperature.
(Zero clinical relevance but 100 nerd points.)
This sets up a neat cycle of constant evaporation and condensation that will eventually reach an equilibrium, where the rate of both processes are equal.
Now if we put this liquid in a container, the vapour phase above the liquid is going to exert a pressure on the walls of the container, as any gas would. This pressure is going to be highest, when the air is absolutely saturated with the vapour of our chosen liquid, at equilbrium.
This pressure is therefore called the saturated vapour pressure.
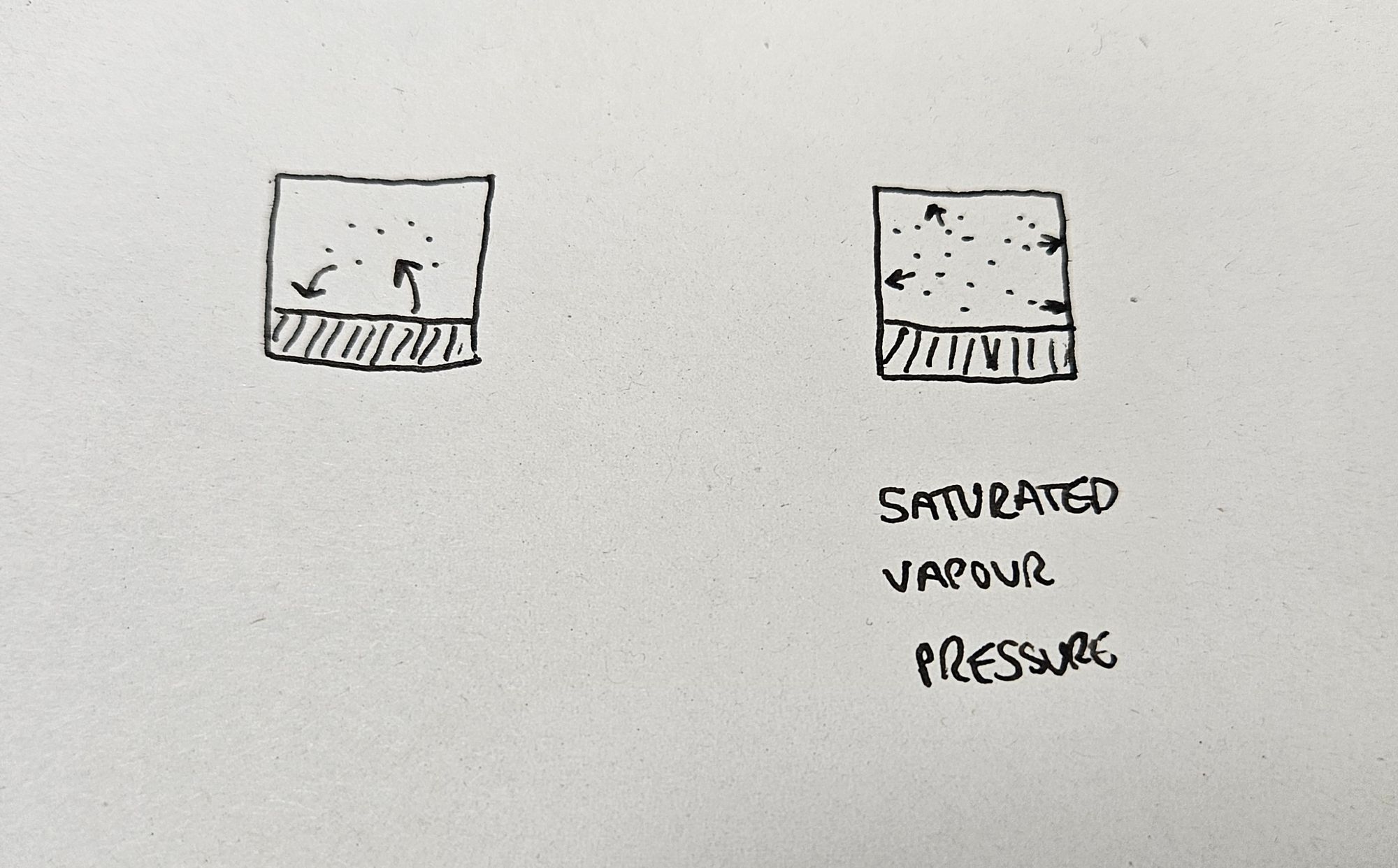
Each liquid has its own saturated vapour pressure, and the more volatile the liquid, the higher this saturated vapour pressure will be.
It doesn't matter what else is in the gas mixture above the liquid, or what pressure it is sat at, the saturated vapour pressure for the liquid doing the evaporating remains consistent as it just refers to the pressure exerted by the the molecules of our liquid that have evaporated.
SVP and Temperature
Now let’s add some heat to the system, and see what happens.
As we apply heat energy to our liquid, a greater number of molecules will possess enough energy to evaporate. And, because there’s more energy in the system, fewer molecules will condense back into liquid.
This means more molecules will be evaporating than condensing, and far more molecules will be in the vapour phase than before. These molecules will naturally therefore exert a greater pressure on the walls of the container, and so it becomes clear why saturated vapour pressure increases with increasing temperature.
Some saturated vapour pressures to be aware of
These are all values for SVP at 20°C
- Sevoflurane - 21 kPa
- Enflurane - 23 kPa
- Isoflurane - 33 kPa
- Halothane - 32 kPa
- Ether - 59 kPa
- Desflurane - 88 kPa*
*You might have noticed this is rather close to atmospheric pressure (101.3 kPa).
By definition, if a substance's saturated vapour pressure is higher than atmospheric pressure, it will boil. That's what the boiling point is defined as - the temperature at which the SVP exceeds atmospheric pressure.
For water at sea level, this temperature is 100°C, but at the top of Everest, it's 68°C, and on Mars it can boil at 0°C.
Desflurane is already pretty close to boiling point at 20°C (meaning it is super volatile) and this is why Desflurane needs a special vaporiser, because it would produce insanely high concentrations of anaesthetic agent if used in a normal vaporiser.
What does a vaporiser do?
Our sevoflurane comes as a liquid, and we want to administer it to the patient as a vapour.
A vaporiser allows a controlled, consistent and reproducible amount of anaesthetic volatile agent to be safely and reliably added to the fresh gas flow of an anaesthetic breathing circuit.
Now, you can't just saturate the fresh gas flow with sevoflurane and give it to the patient, because the saturated vapour pressure of sevoflurane at 20°C is 21kPa.
Given atmospheric pressure is just north of 100kPa, this mixture would therefore be 21% sevoflurane, which is dangerously high, so we need to dilute it down to a more user-friendly concentration for our patient to inhale.
There are two ways of achieving this
Variable bypass
- A controlled proportion of the fresh gas flow is allowed through the vaporising chamber in order to become fully saturated with anaesthetic agent
- This is then diluted back into the rest of the fresh gas flow to give a much lower final concentration
E.g. Sevoflurane in a TEC 5
Measured flow
- The vaporiser heats the liquid into vapour form, and measures how fast the fresh gas is flowing past
- Vapour is then injected directly into the fresh gas flow at the correct concentration based on the measured flow rate of the fresh gas
E.g. Desflurane in a TEC 6
Great, so we have a device that lets us control how much anaesthetic agent we're giving to the patient.
So how much do we give?
Minimum Alveolar Concentration
Here's our explanation to the first question you get asked when you express even the slightest interest in anaesthesia - "Can you tell me what MAC is?"
- Volatile anaesthetic agents work on receptors in the brain, but it's rather tricky to measure concentrations or partial pressures of vapours in brain tissue
- These agents travel to the brain via the blood, but again - tricky to measure
- These agents get to the blood via the lungs
Finally - something we can measure fairly easily.
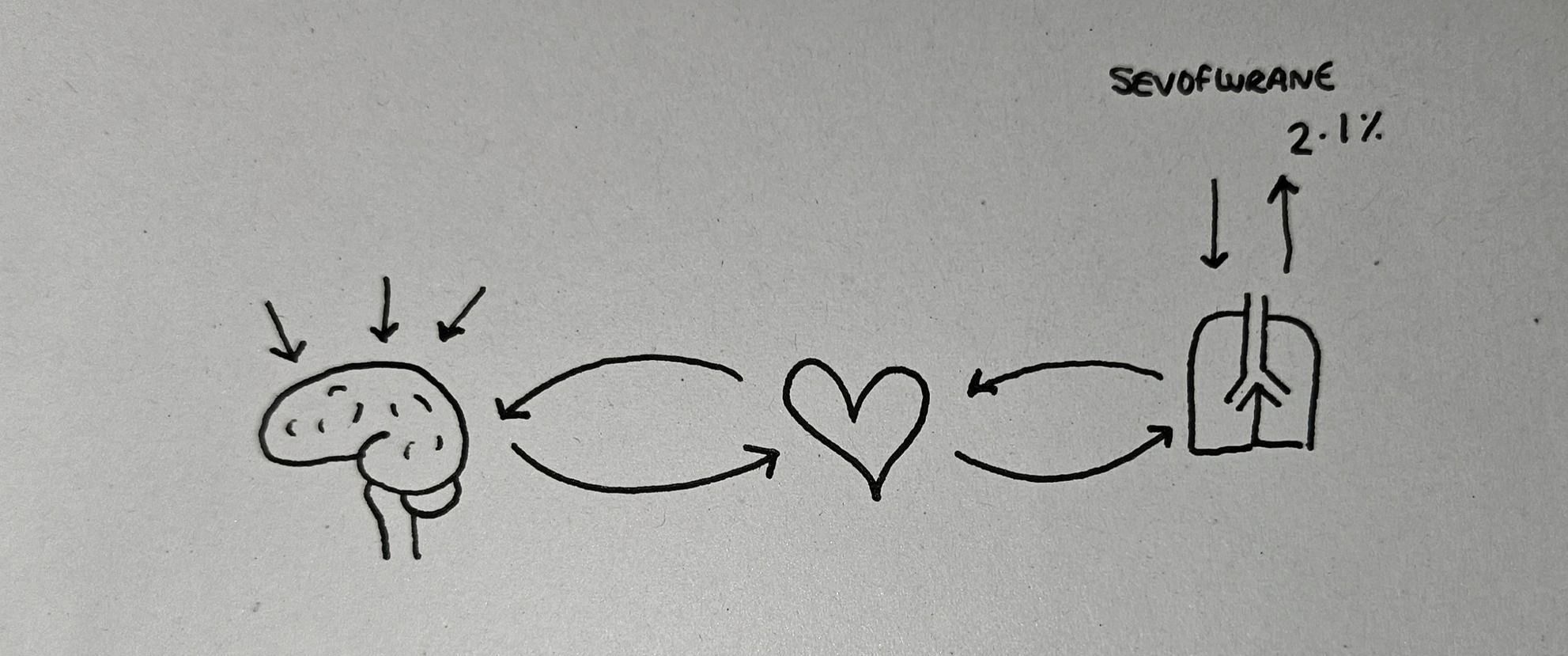
- To begin with, we give the patient anaesthetic agent (sevoflurane for example) via the lungs and it diffuses across into the blood, and then gets shipped to the brain
- Because they've only just started inhaling the sevoflurane, there's a large partial pressure gradient from lungs to blood, and blood to brain, so very little gets transported back to the lungs
- After a while, the brain fills up with sevoflurane, then the blood fills up, and so the excess gets taken back to the lungs and is exhaled
- Once this equilibrium has been established, if 2.1% of the gas mixture exhaled by the patient is sevoflurane, then they have enough sevoflurane in their blood and therefore the brain to ensure they're asleep enough for an operation
This is the minimum alveolar concentration for sevoflurane - 2.1% - and each volatile agent has its own unique MAC value.
The definition of MAC
- When we say MAC we usually refer to MAC50
- The minimum alveolar concentration is the exhaled percentage concentration of anaesthetic agent at which fifty percent of patients will not move in response to a 1cm surgical skin incision
Wait what - why only half? Surely we want everyone to be asleep?
- There are different levels of ‘being asleep’ or depths of anaesthesia
- You can be fast asleep at home but if someone tickles your nose you may well swipe a hand up and swat them away without waking up, and you’d never be aware that you moved at all - you're still asleep
- So you can appreciate therefore, just how deeply asleep you need to be to not even flinch when stabbed with a scalpel
- Furthermore this is without any other pain relief or anaesthesia, and certainly without any muscle relaxant
- So the other 50% of people definitely aren't awake, they just might flinch slightly in their sleep, in the absence of any additional analgesia or muscle relaxation (what we like to call balanced anaesthesia)
So it's okay, really.
Common MAC values
- Methoxyflurane - 0.16
- Chloroform - 0.5
- Halothane - 0.75
- Isoflurane - 1.17
- Enflurane - 1.63
- Sevoflurane - 2.1 (some texts say 1.8, exams usually want 2.1)
- Ethyl ether - 3.2
- Desflurane - 6.6
- Xenon - 72
- Nitrous Oxide - 104*
*WHAT. How can you have 104% Nitrous?!
We keep mentioning partial pressure and concentration. Partial pressure is what matters, but concentration is what the machinery measures, hence the confusion.
The MAC of sevoflurane is actually 2.1kPa, but because ambient pressure is basically 100kPa, this translates seamlessly to the 2.1% we know and love.
The MAC of Nitrous oxide is 104kPa, which is tricky to achieve normally, but in a hyperbaric chamber you could give someone entonox at 2 atmospheres of pressure, with 100kPa of nitrous and 100kPa of oxygen, and they would be exceedingly unconscious.
Simple.
The Vaporising Chamber
Rather than some evil contraption out of Battlestar Galactica, this is simply the box inside the vaporiser where the vapour mixes with the fresh gas flow.
The vaporiser's output is always equal to the saturated vapour pressure of the volatile agent.
The only reason that twisting the dial on the front results in more or less vapour being delivered to the patient is because it's allowing more or less fresh gas to pass through the vaporiser and become saturated, but any gas leaving the vaporiser is always fully saturated at the SVP of the agent.
- For sevoflurane this is 21 kPa at 20°C
This means that (assuming that ambient pressure at sea level is 100kPa) each 100ml of gas leaving the vaporising chamber contains 21ml of sevoflurane vapour, or 21%.
This is clearly a dangerously high concentration of sevoflurane for the patient to breathe, so we need to dilute it down a little.
When you twist the dial to the number 2 on the vaporiser, you're adjusting the gas flow such that only around 10% of the total fresh gas flow is being diverted through the vaporiser.
That 10% becomes fully saturated (with 21 kPa sevoflurane) before being mixed back in with the other 90%, giving a final concentration of around 2.1% sevoflurane.
Easy.
Drawover vs Plenum
You need to know this for the exam, but unless you end up working in the military, you're unlikely to use a drawover vaporiser in clinical practice.
Drawover
- Essentially a pot of liquid that the patient 'draws' air over the top of
- Low resistance
- Lower efficiency
- Not one way
- Not agent specific
Plenum
- These have wicks and baffles to fully saturate a designated proportion of the fresh gas flow with anaesthetic agent
- High resistance, so need to be 'out of circuit'
- Highly efficient
- One way
- Agent specific

Temperature control
The reason I started off by talking about saturated vapour pressure is so we can appreciate the fact that if the temperature inside the vaporiser increases, then the saturated vapour pressure, and therefore the amount of volatile agent leaving the vaporising chamber will increase as well, and ruin our carefully balanced anaesthetic.
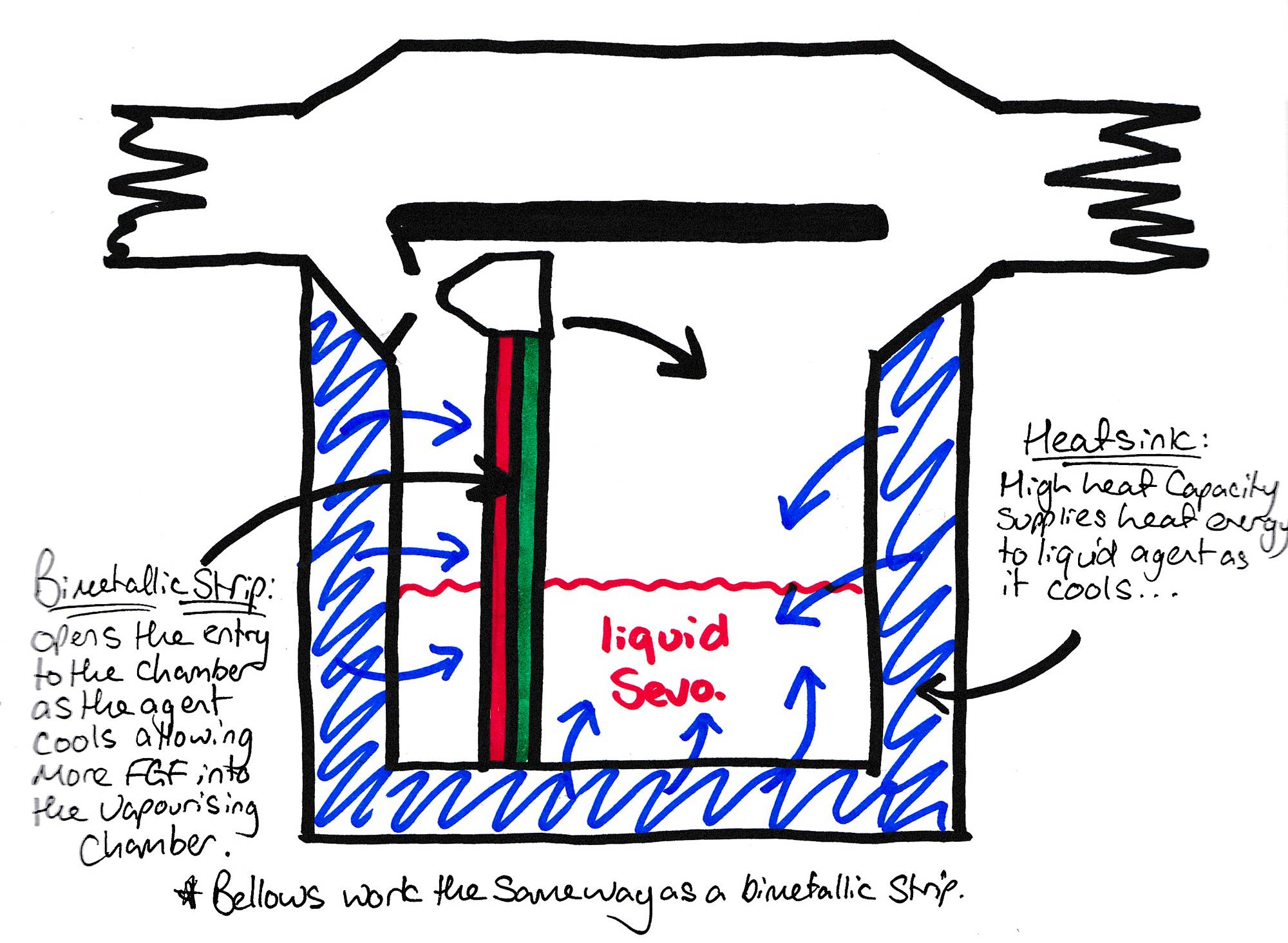
To counter this, vaporisers classically employ several mechanisms:
- A heat sink - to absorb the heat energy of any temperature increase and keep the vaporising chamber at a constant internal temperature
- A bimetallic strip - this is literally a strip with two different metals stuck together. As it warms or cools, the metals expand or contract to different extents, and so it bends. Attach it to a valve and you can automatically change the proportion of fresh gas flow entering the chamber to compensate for the change in temperature. If it gets warmer, and the SVP increases, less flow is needed through the chamber, if the temperature decreases and the agent is less volatile, more fresh gas will need to pass through to reach the same final concentration
- Fancy computer work - nowadays many of the newer vaporisers have complex microprocessor-managed injection technology and active heating that makes the previous two features somewhat redundant, but many machines still employ the good old Tec5s and the exams don’t evolve particularly quickly either, so you definitely need to know about them.
This leads us nicely to an FRCA Primary exam favourite:
How can you classify vaporisers?
As always, there are numerous ways of classifying anything, so the following are our suggested options:
You can classify a vaporiser based on a number of features:
- How the vaporiser regulates the delivered concentration of an agent
- How it actually vaporises the agent
- How it manages temperature changes
- How much resistance it offers to gas flow
- Its position within the circuit
In terms of how a vaporiser regulates the concentration of an agent, your two options are either a variable bypass or a measured flow system.
- Variable bypass - the vaporiser splits the fresh gas flow, and then varies how much of it bypasses the vaporising chamber and thus how diluted the vapour becomes
- A measured flow system is a bit more complex, and it’s what is found in the TEC6 desflurane vaporiser
- With a measured flow system, there is a separate stream of vapour carrying gas that is added to the fresh gas flow
- To ensure it’s adding the right amount, the vaporiser must measure the fresh gas flow, and adjust the vapour addition rate accordingly
Lets zoom in on our variable bypass vaporisers. These can either be of the Plenum or Draw over variety, and you’re much more likely to encounter the plenum type in your day to day practice.
A Plenum vaporiser uses a complex system of wicks and baffles to maximise surface area and ensure the fresh gas flow spends enough time in the chamber to become fully saturated, even at high fresh gas flows. An example is the TEC vaporisers you’ll commonly see in theatre. Examples of draw over vaporisers are Goldman and Oxford Miniature vaporisers.
The main difference between the two is Plenum vaporisers are more accurate but they need the fresh gas flow to be significantly higher than atmospheric pressure because they provide substantial resistance to the gas flow, while drawover vaporisers have much lower resistance to flow and can simply use the patient’s own respiratory effort, which makes them useful for compact, portable systems where pressurised fresh gas flow isn’t available, such as in military scenarios. For your exam, this means that the plenum vaporiser is an out of circuit vaporiser, because the fresh gas flow the patient breathes doesn’t all pass through the vaporising chamber, while a drawover vaporiser is an in circuit vaporiser, as the patient is essentially breathing fresh gas through the vaporising chamber.
Drawover vaporisers can often be used for multiple volatile agents, while each plenum vaporiser is calibrated only to one agent, such as sevoflurane or isoflurane
For those pesky MCQs that they like to spring on you, I’ll repeat the salient points
- TEC5 vaporisers are plenum vaporisers, which are a subset of variable bypass vaporisers
- Goldman and Oxford miniature vaporsiers are drawover vaporisers, which are also a type of variable bypass vaporiser
- The desflurane Tec6 vaporiser is a measured flow vaporiser
The last main difference between vaporisers is how they manage changes in temperature. Plenum vaporisers generally have thermocompensation as we have described previously, in the form of a heat sink and a bimetallic strip, while the TEC6 desflurane vaporiser actively heats the desflurane to 39 degrees celsius, thereby rendering atmospheric changes in temperature relatively redundant.
What are the features of an ideal vaporiser?
The reason they ask ‘ideal’ questions in the viva is to highlight your knowledge of the flaws with the current systems available, for example our current vaporisers provide high resistance, and if you tip them upside down, the sevoflurane spills out into the bypass chamber and causes all sorts of calibration issues.
So here we go:
- An ideal vaporiser should be accurate and reliable, economically viable, and provide low resistance to gas flow
- In terms of reliability, it should be unaffected by changes in fresh gas flow, how much liquid is left in the vaporising chamber, ambient temperature and pressure, or changes in patient ventilation and changes in airway pressure*
- In terms of economic viability, it should be cost effective to manufacture and maintain, and should use vapour efficiently with minimal wastage
- It should also be durable, corrosion resistant and require minimal maintenance
- And you should be able to turn it upside down without worrying about the liquid inside
*The 'pumping' effect occurs when increased airway pressure compresses the gas exiting the vaporising chamber, and forcing volatile out through the inlet as well as the outlet. This leads to an erroneously high concentration of volatile being delivered to the patient.
The aladin cassette
We’ll briefly mention the aladin cassette as these are becoming increasingly commonplace in anaesthetic rooms around the country, and may be examinable in the near future.

Long story short, they’re a hybrid of variable bypass and measured flow and have the following features:
- An agent-specific vaporising chamber (which is the cassette that you click into the machine)
- A microprocessor in the anaesthetic machine itself
The cassette has synthetic lamellae which maximise surface area and ensure the carrier gas is fully saturated, and then the microprocessor measures the fresh gas flow, and adjusts how much passes through the vaporising chamber. The microprocessor also measures the temperature and the pressure within the cassette and adjusts flow for these factors as well. If needed the machine has a fan beneath the cassette and can adjust the temperature as required.
- It allows carrier gas into the vaporising chamber, and then measures the fresh gas flow, using a throttle valve to ensure the correct amount of vapour-saturated gas is allowed out of the cassette into the breathing circuit
- It measures the temperature of the liquid agent, calculates the SVP, and then compensates for this by allowing more or less carrier gas out of the cassette
- It also has metal plates in the base of the cassette and can redirect heat from the anaesthetic machine itself to warm the cassette if needed
- It has a mixing chamber for the carrier gas and bypass gas, to overcome the issues of backpressure and to prevent pumping
Syllabus
- PC_BK_21 Physics of vapours
- PC_BK_33 Vapour pressure: saturated vapour pressure
- PC_BK_34 Vaporisation: process of vaporisation
- PC_BK_35 Vaporisers: principles, including plenum and draw-over, temperature compensation, concentration
Useful Tweets and Resources
References and Further Reading



Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.


