Foetal Circulation
Take home messages
- The foetus somehow thrives in an acidic, hypoxic environment
- The foetus receives oxygen via the ductus venosus, not the pulmonary circulation
- More oxygenated blood is preferentially diverted to the myocardial and cerebral circulation
Imagine this
You've built a plumbing system complete with pumps, valves, pipes and all sorts of goodies, and have been told that your system has to pump fluid from point A to point C, via point B, without stopping or failing for approximately seventy years.
Tough stuff.
Now imagine that for the first nine months of those approximately seventy years, your same system will be attached to the plumbing system built by the last person to attempt this challenge.
Furthermore, your system has to handle the fact that the fluid is now coming from point D instead, and you have to find a way to divert everything past point B in order to reach point C.
Seriously tough stuff.
Finally at the end of those nine months, someone is going to violently rip your plumbing system off the aforementioned pre-existing system, and your system now has to automatically revert to the original task of A to B to C without leakage, stopping or failure.
Essentially impossible.
Well this is what the foetal circulation has to go through every time a new baby is born, so I think that deserves a round of applause.
*claps quietly alone in library
The set up
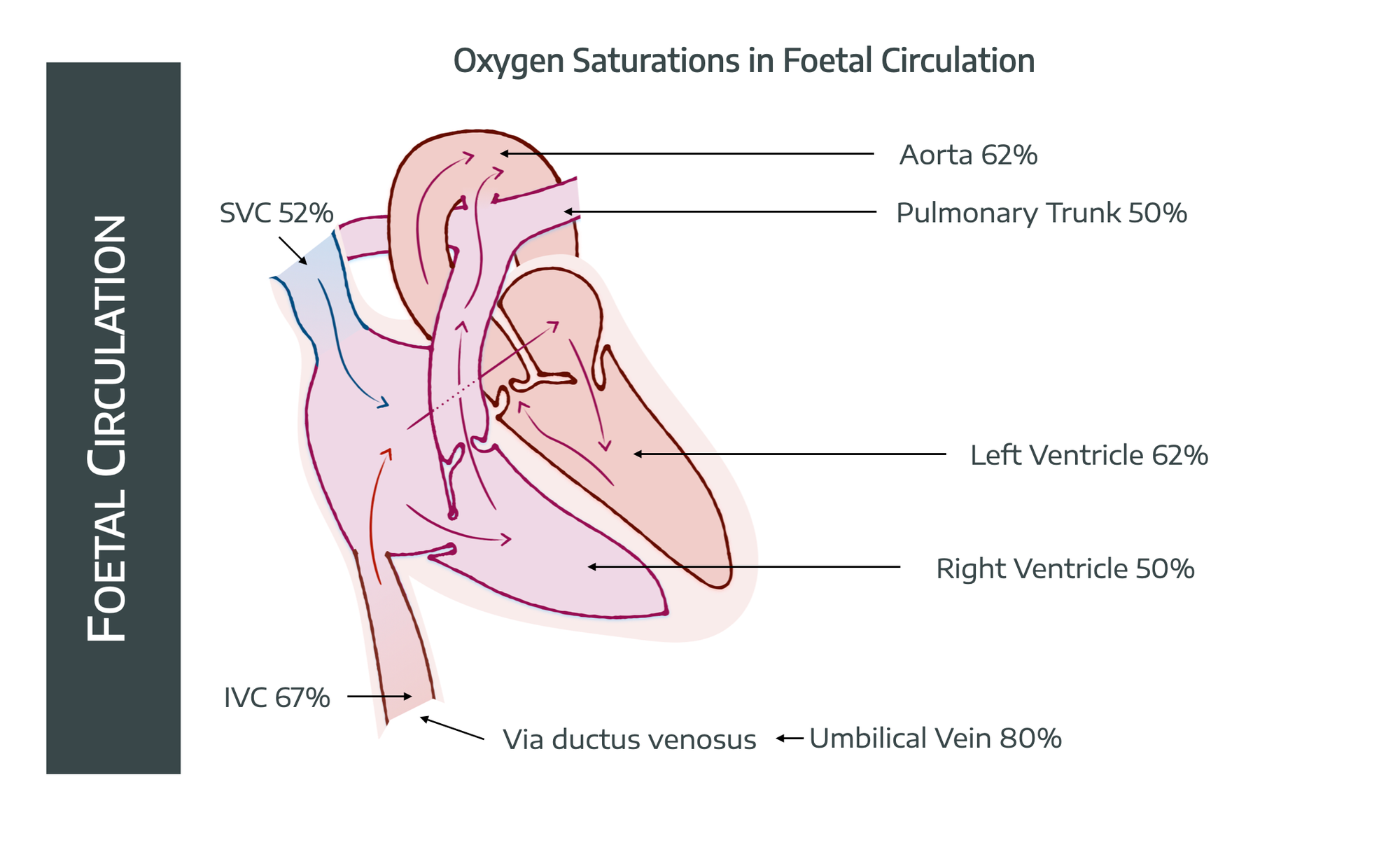
Start with your normal adult heart and blood vessels.
- Now drill a hole between the two atria
- Then plumb a bypass channel from pulmonary artery to aorta
- And another from the umbilical vein past the liver to the IVC
Bingo - foetus mode.
How it works
Start with the baseline premise that the oxygen is now being delivered from the placenta, via the umbilical vein.
Remember the foetal haemoglobin has a greater affinity for oxygen than its adult counterpart, and that saturations of 80% will occur at around a PvO2 of 4 kPa.
Yes - the most oxygenated blood the foetal circulation will ever see is at 4 kPa.
Now ideally the foetal brain is receiving blood with a partial pressure of at least 3 kPa which would correspond to saturations of 60-65%.
So we need to somehow get the blood from placenta to brain, and only lose 1 kPa or 15% saturation in the process.
Challenge accepted.
To achieve this, a terribly clever set up involving shunts, holes and functional valves allows the system to handle the fact that the lungs are collapsed and full of fluid, and all the oxygen is apparently coming from the liver.
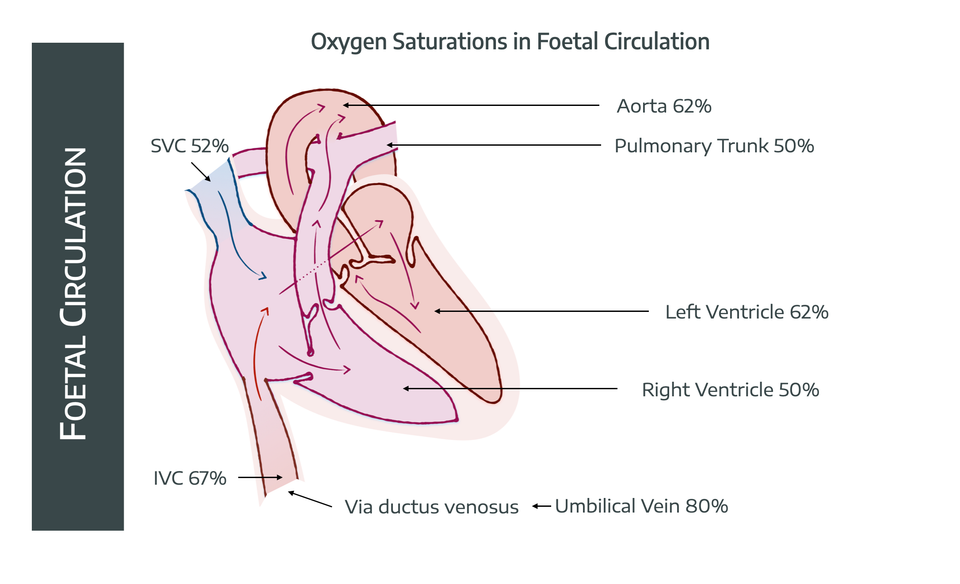
Problem number 1
The somewhat oxygenated blood in the umbilical vein will hit the liver, and all of that precious oxygen will be immediately taken up by the hungry hepatocytes.
To avoid this, the ductus venosus bypasses the liver, carrying the blood straight to the IVC, where it mixes with seriously deoxygenated blood (25-40% sats) returning to the heart from the portal circulation.
- The saturations now drop to 67%
Problem number 2
The deoxygenated blood returning from the head in the SVC (52% saturated) is going to mix with our 67% saturated blood, thereby dragging the saturations down even further.
To prevent this, a glorious crescent-shaped fold of endocardium called the Eustachian valve sits at the junction of the IVC and the right atrium, dutifully funnelling blood from the IVC preferentially through the foramen ovale to the left atrium, rather than into the right ventricle.
- This means that by the time it reaches the left ventricle, and then the aorta, and the coronary arteries, the saturations have only dropped to around 62%
- With only a short journey left to the brain, we now have an uncomfortable-but-sufficient oxygen content for the foetal brain, which hopefully isn't thinking too hard at this stage
The deoxygenated blood from the head and SVC meanwhile, flops lazily through the right atrium into the right ventricle.
- The right ventricle then pumps this into the pulmonary artery, with saturations of around 50% at this point
- 10-15% of this goes to the lungs, the remainder heads on through the ductus arteriosus into the aorta
Crucially, the ductus arteriosus joins the aorta after the cerebrally-destined vessels have already siphoned off some of the redder red stuff, and it is through this anatomical set up that the foetus is able to preferentially oxygenate the head and coronary circulations, leaving the remaining organs to sizzle gently in the hypoxic, acidotic soup that remains.
Since we have two ventricles pumping into the same descending aorta, we talk about the cardiac output in the foetus as the 'combined' cardiac output of both ventricles.
- In the foetus, the right ventricle ends up being responsible for over 60% of the combined cardiac output
- 45% of the combined ventricular output heads straight to the placenta, for hopefully obvious reasons
How does it handle such low oxygen?
While the blood gas measurements in the average foetus would make even the hardiest of adult anaesthetists wince, the foetus has a sneaky trick up its amniotic sleeve - foetal haemoglobin.
- If there's less oxygen available, you need to be better at picking it up and carrying it
- Foetal haemoglobin has a much higher affinity for oxygen, allowing the foetal molecules to rip the O2 molecules off the maternal haemoglobin in the spiral arteries in order to deliver it to the foetal brain and other organs
- There's also lots of it - 160-170 g/L at term
- There's also less 2,3 - DPG than in an adult, driving oxygen uptake further
- The foetus also has a very large combined cardiac output for something so little - 400ml/kg/min - which helps with O2 delivery enormously
- This means even though the partial pressures are low, the haemoglobin is able to make do and carry enough molecules to the brain for the foetus to survive
This does rather put into perspective how important it is to maintain maternal oxygen saturations doesn't it?
The big switch
It's go time.
Baby is squeezed, dragged or sliced into the room whereupon the previously warm and vasodilated umbilical cord feels its first taste of cold room air and protests violently by going into profound vasospasm.
Equally displeased by its unceremonious removal from a nine-month warm bath, baby hopefully has a thoroughly good shout about the whole situation.
These two processes ignite the fuse for the explosive transformation from the foetal to adult circulation, and it's all terribly clever indeed.
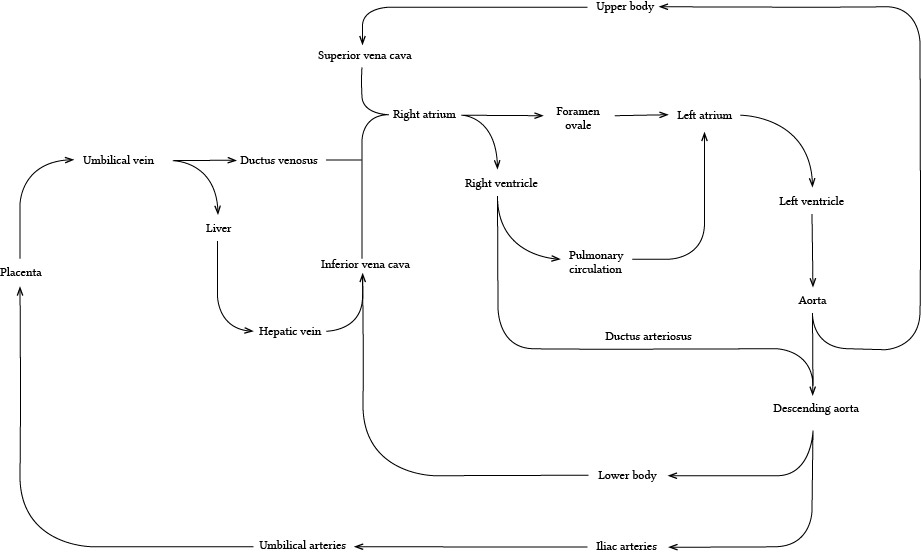
What changes occur in the foetal circulation at the baby's first breath?
- The cord goes into spasm, and may also be clamped
- This increases the systemic vascular resistance felt by the aorta and left ventricle
- Meanwhile baby takes her first breath, and generates negative intrathoracic pressure
- This sucks open the fluid-filled alveoli, and rapidly opens up the pulmonary vasculature
- Crying and grunting serve to recruit the alveoli through gas trapping and maintaining intrinsic PEEP, causing rapid inflation of previously very soggy lungs
- The entry of oxygen into the alveolus undoes the hypoxic pulmonary vasoconstriction and relaxes the blood vessels
- This decreases the pulmonary vascular resistance felt by the right ventricle
- This leads to dramatically increased flow through the pulmonary circulation, and thus to the left atrium
- Meanwhile the clamped umbilical vein is no longer sending blood to the right atrium, causing a drop in right atrial pressure
- The pressure in the left atrium increases compared to the right atrium, and the flap valve of the foramen ovale is slammed shut
This separates the circulation into a higher pressure left, and lower pressure right side.
- With a sudden jump in both preload from the left atrium, and afterload from the clamped umbilical cord, the left ventricle is Frank-Starling'ed into contracting tremendously more vigorously than before
- Pressure in the aorta rises far above that in the pulmonary artery, and hence the previously right-to-left shunt upon which the foetus was dependent now slows, stops and may even reverse in light of a new pressure gradient
There will thus be a small degree of left-to-right shunt through the ductus arteriosus until the point at which it decides to close.
- Functionally this occurs due to profound vasospasm over the next 12 - 24 hours
- Anatomically it fuses to form the ligamentum arteriosum over the next 2 - 3 weeks
Catecholamine and cortisol release during the somewhat stressful birthing process upregulate epithelial Na⁺ channels (ENaCs) in the endothelium of lung blood vessels. These drag sodium out of the fluid in the lungs, and the water duly follows.
This leaves (hopefully) some nicely inflated lungs with a now low pressure, high volume pulmonary circulation, and two separate parallel circulations operating at very different pressures.
Hopefully.
Meanwhile, the humble ductus venosus, receiving far less throughput thanks to baby's new umbilical accessory, slowly begins to close over the next few days to form the ligamentum venosum.
- Around 50% of the blood flowing through the IVC bypasses the liver via the ductus venosus
- If it doesn't close properly, it forms an intrahepatic portosystemic shunt (like an automatic TIPSS procedure)
- This may or may not require sorting out at some point
Why might a baby's circulation revert to the foetal set up?
- Hypoxia, acidosis, hypercarbia and hypothermia will constrict the pulmonary vasculature
- If this happens to a sufficient degree, then the right ventricular and atrial pressures will rise, and venous return to the left atrium will drop
- The foramen ovale and ductus arteriosus have not fully closed yet
- This pressure change can cause the foramen ovale to flap open again, and re-establish right-to-left shunting through the ductus arteriosus
The problem is that there is now no placenta to supply any oxygen, so the foetal set up won't work any more.
- Hence there is more hypoxia
- More acidosis
- More vasoconstriction
- And the cycle continues
Briefly, what happens in structural congenital cardiac conditions?
An enormous topic condensed into a single block of a post. Here goes.
- You either get a left to right (not cyanotic) shunt
- Or a right to left (cyanotic) shunt
Some examples are listed.
Patent ductus arteriosus
- Continued flow through the ductus that has failed to close means much of the aortic output flows back into the pulmonary circulation
- This is inefficient but not cyanotic as the systemic blood is still well oxygenated, there's just less of it
- It can lead to pulmonary hypertension and right heart failure if not dealt with
- The left heart can also fail acutely if it cannot keep up with the demand of essentially supplying both circulations at once
Septal defects
Atrial, ventricular or both
- Blood flows from left to right down a pressure gradient
- Not cyanotic but again, inefficient
- Can lead to congestive heart failure
Tetralogy of Fallot
Technically 'tetrad' as tetralogy specifically refers to literary work, but let's not dwell on the pedantics.
- Overriding aorta
- Pulmonary stenosis
- Ventricular septal defect
- Right ventricular hypertrophy
Doesn't cause problems in utero but once out in the world results in right to left shunt , and therefore cyanotic shunting, and needs surgical correction.
The key point is that cyanotic congenital heart conditions are often duct-dependent, meaning they rely on shunting in order to adequately oxygenate the blood, because the native plumbing isn't able to do so.
Classically these present with respiratory distress and hypoxia in the hours to days after birth as the ductus arteriosus closes and the duct-dependency makes itself painfully apparent.
Transposition of the great vessels
- The aorta comes off the right ventricle
- The pulmonary artery comes off the left
- The shunts (foramen ovale and ductus arteriosus)work overtime to keep the blood oxygenated
- As soon as the shunts stop, you have two disconnected parallel circulations, with no way to oxygenate the brain
Baby needs an ASD, VSD or PDA to survive long enough to undergo surgical correction.
The easiest way to achieve this is with a prostaglandin infusion. A slightly less simple option is a balloon atrial septostomy to force the foramen ovale open and allow mixing of atrial blood from both chambers.
Some unexaminable points of interest
We say ‘unexaminable’ fully aware that the FRCA has the wherewithal to examine whatever it damn well pleases.
- The foetal circulation runs a MAP of around 40 mmHg, which is surprising for such a small circulation with tiny vessels that offer naturally higher resistance to flow
- The combined ventricular output of the foetus per kilo would be around 30 litres per minute in an adult
- The right ventricle does 60% of the work when it comes to the combined ventricular output
- The Frank-Starling mechanism does work in the foetal heart, but over a terribly narrow range of preload
- This makes sense in a situation where the whole system is hugely vasodilated and there's an instant source of oxygenated intravenous fluid available should the need for more preload arise
Here's a painfully simplified video
References and Further Reading

Primary FRCA Toolkit
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.


