How to read an ABG

If I had a sick patient in front of me, and I was only allowed to have one test to help me figure out what was going on, I'd choose an ABG.
It is an enormously useful and rapidly avaible source of crucial information that can help you not only to diagnose the patient's problem but also to help guide your management.
But if you're not used to interpreting the numbers then it can be rather daunting as to what they mean, and what you should do next.
But once you get the hang of it, it's an easy and reliable way to get a rapid idea of how well, or not, your patient is doing.
So here's my 5 step approach to interpreting what on earth is going on.
Watch the video if you prefer
@anaestheasier How to interpret ABG or arterial blood gases
♬ original sound - Anaestheasier
Step 1 - Oxygen
Start with what would kill the patient first - hypoxia.

The reference level is usually around 11-15kPa, and dangerous hypoxia would be anything below 8kPa.
This patient was having a severe asthma attack, but had been given about 80% oxygen with his nebulisers, so his oxygen levels weren't actually too bad.
In this trend of repeated blood gases you can see he started out on high flow oxygen through a facemask prior to intubation, whereupon his oxygen levels increased dramatically as he was no longer struggling to breathe because we were ventilating him mechanically.
We then turned down the inspired oxygen from 100% to 45%, because too much oxygen is harmful to the lungs, and his blood oxygen level settled to a more normal number accordingly.
Step 2 - pH
pH is defined as the inverse logarithm of the concentration of hydrogen ions.
This means more ions = smaller pH
Your enzymes and proteins only work properly in a narrow range of pH, so the blood's pH is very tightly controlled by all sorts of buffer systems and feedback mechanisms to keep it within the normal range.
The normal range is 7.35 to 7.45
- Less than 7.35 is an acidaemia
- More than 7.45 is an alkalaemia
Why do I say 'acidaemia' rather than 'acidosis'?
Many people just use 'acidosis' or 'alkalosis' when they see the pH of the blood - which isn't technically correct.
The acidosis is the underlying problem, while the acidaemia describes whether or not the blood has a lower pH than normal.
You don't need an acidaemia to have an acidosis.
You can have an underlying problem (an acidosis or an alkalosis) that would lead to a change in blood pH (an acidaemia or alkalaemia) if nothing was done to correct it.
However if you have some form of compensation then the pH of the blood can be normal, even though the underlying process hasn't gone away.
So you can have a respiratory acidosis with a normal blood pH if there is a compensatory metabolic alkalosis to drag that pH back up to the normal range.
The pH is now normal, they don't have an acidaemia anymore, but the acidosis is very much still happening.
Step 3 - Carbon Dioxide
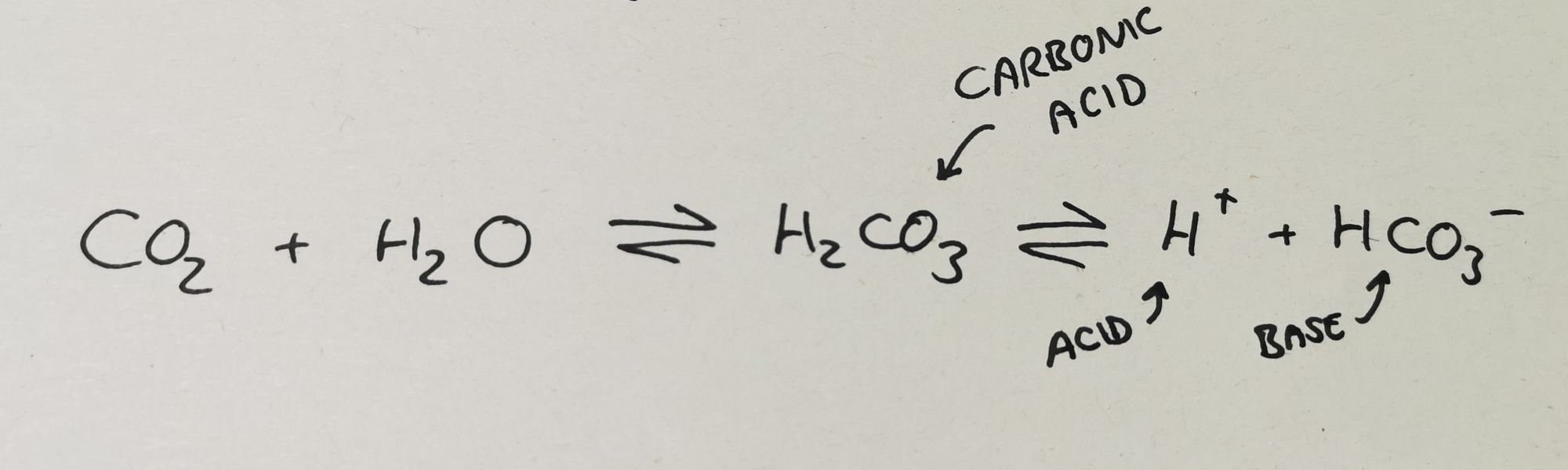
When CO2 is produced by cells in the body, it dissolves into the blood and some of it reacts to form carbonic acid.
This then dissociates into hydrogen ions and bicarbonate ions.
CO2 is almost entirely controlled by respiratory function
- More breathing => more CO2 removed, fewer hydrogen ions produced
- Less breathing => less CO2 removed, more hydrogen ions produced
This is why we call it a respiratory acidosis or a respiratory alkalosis

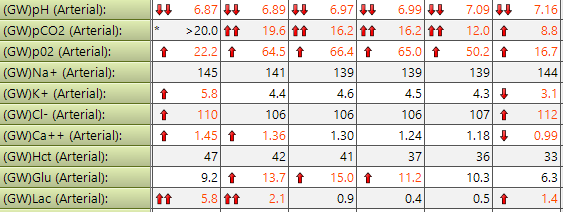
In the image above, you can see that the degree of acidaemia (how low the pH is) maps nicely with how high the CO2 is.
This is an example of severe type II respiratory (or ventilatory) failure, leading to CO2 retention and a respiratory acidosis. As the patient's ventilation improves from left to right, the CO2 starts to drop, and the acidaemia starts to normalise.
This acute respiratory acidosis is uncompensated, because there is a severe acidaemia.
Step 4 - Bicarbonate
Bicarbonate is a very important ion buffer in the blood. It counteracts the H+ ions to help maintain that all-important pH of 7.35-7.45
A normal bicarbonate is around 22-28mmol per litre.
If there is a process in the body resulting in an acidosis, then the bicarbonate ions will be 'mopped up' as they go round scavenging the excess H+ ions.
This means the measured HCO3- levels in the blood will drop, so a low bicarbonate is an indicator of a metabolic acidosis.
Examples of causes of metabolic acidosis include:
- Sepsis
- Ischaemia
- Renal failure
- Poisoning
Equally an excess of bicarbonate implies a metabolic alkalosis.
Check out this example

In this blood gas:
- The pH is dangerously low - there's a huge acidaemia
- But the CO2 is low which would produce a respiratory alkalosis, so something else must be going on
- The bicarbonate is very low (normal is 22-28) producing a metabolic acidosis, which does explain the acidaemia
- So this is a metabolic acidosis, leading to a severe acidaemia
But why is the CO2 so low?
Step 5 - Compensation
As we mentioned earlier, the body really wants to hold the pH in that normal 7.35-7.45 range, and to do this it has all sorts of complex mechanisms in place to compensate for when things go wrong.
These processes don't fix the underlying problem, they just patch over the holes temporarily by making the pH better.
So if a patient has a severe metabolic acidosis, they are likely to compensate by removing CO2 (to produce a respiratory alkalosis) which will then bring the pH back up to normal.
This is called Kussmaul breathing or air hunger, and we see it in conditions such as Diabetic Keto Acidosis.
That deep, sighing breathing is characteristic of a patient with a metabolic acidosis who is trying to compensate by reducing the level of CO2 in their blood.
If we look back at our blood gas:

We can see the PaCO2 is very low at 2.48, which will produce a significant respiratory alkalosis.
The patient's blood, however is acidaemic, meaning there must be an acidosis occuring somewhere. Since the respiratory component is doing the opposite it must be a metabolic acidosis that is the underlying problem - which is consistent with the bicarbonate of 4.7mmol per litre.
This therefore is a metabolic acidosis with respiratory compensation, but given the blood is still highly acidaemic, the compensation is said to be partial, because despite its best efforts, the respiratory system hasn't managed to normalise the blood pH.
Clear as mud?
Hopefully this makes sense, but don't feel disheartened if you have to go over the subject a whole bunch of times before it sticks - it's a tricky concept to understand.
Let us know if you have any questions either by commenting or emailing anaestheasier@gmail.com, and there are some more useful resources below if you would like to learn more!
Useful Resources
The level you need to know for medical school

If you'd really like to get your nerd on


