Acute on Chronic Liver Failure

Take home messages
- There's acute liver failure, chronic disease with decompensation, and there's acute-on-chronic liver failure
- They key difference is the increase in mortality
- Severity scoring can help guide treatment decisions
What is it?
Well I'll tell you what it's not:
- It's not acute liver failure
- It's not decompensated chronic liver disease
It's something entirely (but not really) different.
They're based on the same idea (chronic liver disease with acute problem) but the key distinguishing feature between acutely decompensated chronic liver disease and ACLF is the speed of progression to organ failure, and the resulting mortality.
You can acutely decompensate your chronic liver disease and have no organ failure and a mortality at 28 days of less than 5%, but if you have ACLF then that number jumps to 20%, and up to 70% if you have more than two organs failing.
- Around 15% of patients with cirrhosis will decompensate per year
- Up to a third of these will develop ACLF
What are the hallmark features of ACLF?
They present much like any other chronic liver patient having a bad day - but the key is they often have multiple organ involvement from the start, and get very unwell very very quickly compared to a standard decompensation of chronic liver disease.
How bad is it?
We love a good scoring system, particularly in liver disease where we're spoilt for choice:
- Fatty liver index
- R factor for liver injury
- MELD
- MELD-Na
- NAFLD Fibrosis Score
- NAFLD (Non-Alcoholic Fatty Liver Disease) Activity Score
- Maddrey's Discriminant Function for Alcoholic Hepatitis
- Child-Pugh Score for Cirrhosis Mortality
- Steatosis-Associated Fibrosis Estimator (SAFE) Score
- Liver Decompensation Risk after Hepatectomy for Hepatocellular Carcinoma
- ALBI (Albumin-Bilirubin) Grade for Hepatocellular Carcinoma (HCC)
- Fibrosis-4 (FIB-4) Index for Liver Fibrosis
This isn't even all of them, we're just making an unnecessarily excessive point.
But apparently we needed another calculator, just for ACLF, because none of these accurately predict the mortality associated with this strange condition.
Enter the CLIF-C ACLF Score
This bespoke calculator assesses three main parameters:
- Age
- White cell count
- Organ dysfunction (for kidneys, liver, heart, brain, blood and lungs)
And then gives you a score of how likely they are to have died after 1 month, 3 months, 6 months and 1 year.
Alternatively
The rather unimaginatively named European Association for the Study of the Liver have decided to assign three equally unimaginative grades of severity to ACLF:
- Grade 1 - They just have renal failure, or a 'bit' of renal and something else*
- Grade 2 - They have two organs failing
- Grade 3 - Three organs
*Technically it's renal failure, or a creatinine of 134 - 177 plus liver or heart or blood failure or encephalopathy
But we prefer 'a bit of renal and something else'.
Mortality at 28 days is as follows:
- Grade 1 - 20–30%
- Grade 2 - 30–40%
- Grade 3 - 70–80%
Whichever score you use - more organs = more badness.
Why does it happen?
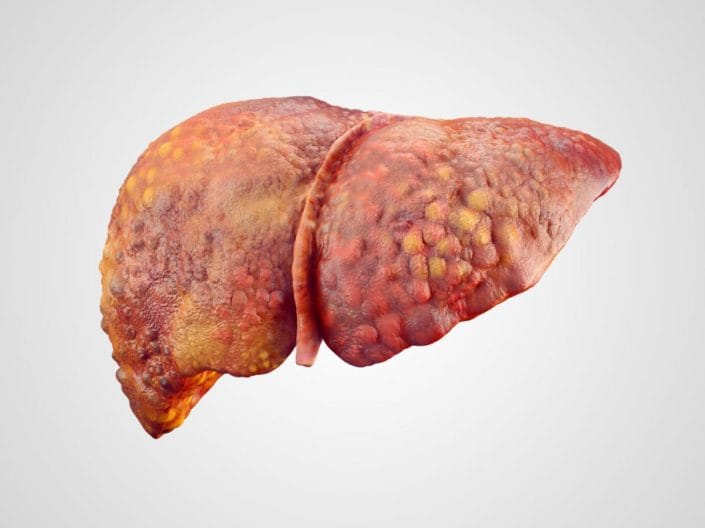
The liver is usually an unfathomably robust organ, capable of immense feats of repair and regeneration, even when half of the damned thing is removed entirely.
However once it becomes chronically diseased, and in particular cirrhosed, it becomes rather more fragile.
This means it only takes a fairly mild trigger to send the already crumbly liver into a spiral of despair, dragging other organs down with it.
You already have a grumbling low-level degree of inflammation throughout the body when the liver is cirrhotic, due to:
- Gut bacteria badness (dysbiosis)
- Leaky intestines (increased permeability)
This means there's more translocation of bacteria across the gut wall into the blood, and therefore more triggering of systemic inflammation and recruitment of angry white cells.
And where does all the blood from the gut go first?
Livertown.
Having irritated the innate immune system and triggered a continuous grating inflammatory response, cirrhosis then takes a swing at the patient's adaptive immune system.
- Continuous exposure to endotoxins reduces the sensitivity of dendritic cells, meaning they don't respond all that well to infection
- Continous metabolic and hormonal derangement
- Throw in high ammonia, low albumin and salt imbalance and the immune system is fighting a losing battle from the start
What are the triggers of ACLF?
- Infection - mainly gram negative stuff like Klebsiella and Pseudomonas
- Variceal bleeding - carries best prognosis of these options
- Hepatitis - viral, alcoholic or ischaemic
- Cardiac failure with hepatic congestion
- Surgery - particularly ERCP
30% of diagnoses of ACLF have active infection at the time, and this carries worse prognosis than other triggers.
How does this cause organ failure?
- The trigger plus the inflammatory response together damage an already damaged liver just a little more
- This causes necrosis and hepatic inflammation
This in turn leads to:
- Increased portal pressure
- More translocation of gut bacteria
- More inflammation and immune dysfunction
- More arteriovenous shunting, sending this toxic blood around the body to affect all of the other organs
What are the features of acute liver decompensation?
- Encephalopathy
- Ascites
- Variceal bleeding
- Serum bilirubin >205 μmol/Litre
- Coagulopathy
Why is the blood pressure low?
Well to start with you can consider all of the usual reasons you might encounter a low blood pressure:
- Hypovolaemia and bleeding
- Cardiogenic or obstructive shock
- Distributive (septic or anaphylactic) shock
Any of these may be present at the time you see your patient and need to be ruled out promptly.
Liver patients however do tend to run rather low blood pressures on a day to day basis, largely as a result of widespread (and particularly splanchnic) vasodilation as a result of increased hepatic resistance (from the cirrhosis) and inflammation.
This ends up producing a low-resistance system but with relatively decent flow, until it starts to fail, classically presenting with a hyperdynamic circulation that fails to keep up with demand if there's even a smidge of extra inflammation or decompensation.
What cardiovascular changes are seen in ACLF patients?
- Cirrhosis leads to increased intrahepatic resistance and inflammation
- This causes portal hypertension and splanchnic vasodilatation
- There is also vasodilatation elsewhere in the body
- This drops the vascular resistance, and produces a hyperdynamic, low pressure circulation
- There is also an increase in total blood and plasma volume, but most of this accumulates in the hugely dilated splanchnic vessels, meaning the actual circulating volume is relatively low
- This triggers salt and water retention through the renin angiotensin aldosterone system, as well as a compensatory tachycardia
Which fluid to use?
It's a tricky balance between fluid resuscitation to maintain meaningful perfusion and not overloading the patient, and since colloids have now been deemed dangerous and evil, you essentially have two options:
- Balanced crystalloid
- Albumin
Albumin may actually be doubly helpful here, because not only is the albumin low in patients with liver disease, but it's also functionally rubbish.
Subtle changes in structure during its manufacture mean it doesn't bind to drugs, bacterial toxins and reactive oxygen species nearly as effectively, so giving your patient a top up of 'proper' albumin may be doing them more favours than just boosting the blood pressure.
When should I use albumin?
These are the recommendations from the ALPS trial, which took critically ill cirrhotic patients with sepsis and hypotension and tested whether 20% albumin or Plasmalyte was better at reaching a target mean arterial pressure of 65 mmHg at three hours.
- Albumin improved the shock state faster, and lactate improved more quickly
- But albumin also caused more pulmonary complications
- There was also no difference in mortality at 28 days
So there are now four specific situations when albumin is recommended:
- Spontaneous bacterial peritonitis
- Acute kidney injury
- Hepatorenal syndrome associated with vasopressors
- After large volumes of ascitic drainage
If you've given some fluid (or albumin) and their blood pressure is persistently failing to meet a MAP of 65 mmHg then it's time to do what we do best - plumbing:
- Arterial line
- Central line
- Pressors - noradrenaline is first line, vasopressin and terlipressin second
They're bleeding
I'm not surprised.
What with the portal hypertension, the cirrhotic coagulopathy, the delicate distended varices and the platelet dysfunction, it's no wonder that these patients often bleed fairly spectacularly.
What are the transfusion targets in ACLF?
- Hb >70
- Platelets >50
- Fibrinogen >1.5
The tricky thing is that they're also prothrombotic due to a lack of production of the anticoagulant factors in the blood, such as proteins C and S.
Liver patients reach a strange new balance point where a deficiency of both prothrombotic and anticoagulant factors mean they're actually fairly balanced out, albeit with an increased risk of both ends of the bleeding badness spectrum.
The long story short is to talk to gastro and haem, and use TEG or ROTEM to guide your management.
Why have they got AKI?
Because kidney dysfunction is the most common other organ failure seen in acute on chronic liver failure, seen in around half of cases.
It's actually rather tricky to measure as the patient's creatinine is probably a bit off for several reasons:
- Poor muscle mass
- Increased secretion of creatinine in the renal tubules
- Increased dilution by plasma volume
- Bilirubin levels interfere with creatinine measurement
Add on top of that a new AKI and it's quite hard to know how many creatinines is too many creatinines.
Tell me about hepatorenal syndrome
It's nebulous and weird, and you need to rule out other causes first:
- Nephrotoxins
- Intrinsic renal pathology
- Hypoperfusion
If you've done that and you're still looking at rubbish renal function in a patient with liver disease, it might be HRS.
Put simply, the kidneys lose their ability to compensate for the low blood pressure and splanchnic vasodilatation.
This leads to inappropriate renal vasoconstriction, hypoperfusion and AKI.
To manage it:
- Stop nephrotoxics
- Ensure there isn't a blockage
- Fluid resuscitation
- Treat infection
- Hope for the best
Albumin is recommended here, with a loading dose of 1 g/kg, followed by up to 40 g per day.
They should also be given terlipressin 0.5mg QDS as their first line pressor, as it reduces the splanchnic vasodilatation and they seem to respond to it better.
They're confused
So am I.
Neurological dysfunction occurs in hepatic disease as a result of toxin build up - in particular ammonia - which occurs for two reasons:
- The liver isn't clearing the toxins as efficiently
- Portosystemic shunting allows toxins to bypass the liver entirely
How is hepatic encephalopathy graded?
Using the West Haven criteria.
- Grade 1 - alert but strange behaviour
- Grade 2 - drowsy and very disoriented, possible asterixis
- Grade 3 - incoherent and very sleepy, rousable to voice
- Grade 4 - comatose, decorticate or decerebrate posturing
It will come as no surprise, dear reader, that grades III and IV are bad for you, and are thusly independently associated with increased mortality.
You will be told that a normal ammonia level essentially rules out hepatic encephalopathy - this is not true.
Why not?
- Ammonia measurement is incredibly unreliable - venous vs arterial samples, handling, time to lab, haemolysis, fasting state all impact the level in an unpredictable fashion
- Ammonia levels change rapidly
- The reference range for 'normal' is broad (0 - 50 micromol/L)
- Patients with hepatic encephalopathy can sit at the 'upper end' of normal, but be profoundly encephalopathic
By all means use ammonia as another piece of evidence, but remember HE is a clinical diagnosis based on context and pre-test probability, and cannot be ruled out with a single number from a blood test.
Do I need to do neuroprotective ventilation and stuff for hepatic encephalopathy?
Good question, and not really.
Our neuroprotective ventilation strategies usually mitigate against raised intracranial pressure and cerebral oedema, neither of which are usually present in acute on chronic liver failure.
Most of the neurological fixing is done by bringing hyperammonaemia down and hyponatraemia (slowly) back up again.
- Careful fluid therapy
- Lactulose
- Phosphate enemas
Avoid dehydration, treat infection and monitor closely.
They're getting antibiotics right?
Almost certainly.
So yes, a low threshold for antimicrobials is probably a sensible plan in this exceedingly vulnerable cohort of patients.
The most common sources are:
- Pneumonia
- Urinary tract infection
- Spontaneous bacterial peritonitis
Just remember to try and confirm the source, to keep micro happy.
What investigations should I request?
- Blood cultures
- Chest xray (or CT)
- Urine culture
- Ascitic tap
- Pleural tap
- Sputum samples
So what are my management principles for acute on chronic liver failure?
- Resuscitate as normal - ABC
- Try and identify (and treat) a cause - antibiotics, hepatotoxic drugs
- Support organs that need help - ventilation, RRT, vasopressors
- Cautious volume resuscitation - use albumin when indicated
- Early referral to intensive care - use CLIF-C score to help with decisions
- High protein nutrition - unless severely encephalopathic
- Transplant referral where appropriate
Useful Tweets and Resources
Acute on chronic liver failure (ACLF) - beautifully summarised in the 🆕 EASL guideline #LiverTwitter https://t.co/RaBmhJj9xv pic.twitter.com/6dSwuNxtgp
— Keith Siau (@drkeithsiau) June 29, 2023
💉 Albumin isn’t just volume replacement in acute on chronic liver failure.
— British Journal of Anaesthesia (@BJAJournals) September 11, 2025
It binds toxins, reduces oxidative stress & may modulate inflammation.
Used in SBP, HRS, AKI, & post-paracentesis.
Evidence is evolving - read more in the BJA Education: https://t.co/fEOvmIXTVJ pic.twitter.com/2NbWEXu1xR
References and Further Reading

Primary FRCA Toolkit
While this subject is largely the remit of the Final FRCA examination, up to 20% of the exam can cover Primary material, so don't get caught out!
Members receive 60% discount off the FRCA Primary Toolkit. If you have previously purchased a toolkit at full price, please email anaestheasier@gmail.com for a retrospective discount.

Discount is applied as 6 months free membership - please don't hesitate to email Anaestheasier@gmail.com if you have any questions!
Just a quick reminder that all information posted on Anaestheasier.com is for educational purposes only, and it does not constitute medical or clinical advice.
Anaestheasier® is a registered trademark.


